In Development this week (Vol. 140, Issue 18)
Posted by Seema Grewal, on 27 August 2013
Here are the highlights from the new issue of Development:
FGF10 function in the lung branches off
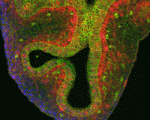 Lung development in mice involves specification of the primary lung field followed by the formation of lung buds, which subsequently undergo outgrowth and branching morphogenesis to form the stereotypic bronchial tree. Localised expression of Fgf10 in the distal mesenchyme adjacent to the sites of lung bud formation has long been thought to drive branching morphogenesis in the lung but now, on p. 3731, Stijn De Langhe and colleagues challenge this model. They show that lung agenesis in Fgf10 knockout mice can be rescued by ubiquitous overexpression of Fgf10, demonstrating that localised Fgf10 expression is not required for lung branching morphogenesis in vivo. Instead, they report, localised Fgf10 prevents the differentiation of distal epithelial progenitors into Sox2-expressing airway epithelial cells, thus suggesting that Fgf10 plays a role in proximal-distal patterning. Furthermore, they show that, later in development, Fgf10 can promote the differentiation of airway epithelial cells to basal cells, a finding that has important implications for understanding and improving lung injury and repair.
Lung development in mice involves specification of the primary lung field followed by the formation of lung buds, which subsequently undergo outgrowth and branching morphogenesis to form the stereotypic bronchial tree. Localised expression of Fgf10 in the distal mesenchyme adjacent to the sites of lung bud formation has long been thought to drive branching morphogenesis in the lung but now, on p. 3731, Stijn De Langhe and colleagues challenge this model. They show that lung agenesis in Fgf10 knockout mice can be rescued by ubiquitous overexpression of Fgf10, demonstrating that localised Fgf10 expression is not required for lung branching morphogenesis in vivo. Instead, they report, localised Fgf10 prevents the differentiation of distal epithelial progenitors into Sox2-expressing airway epithelial cells, thus suggesting that Fgf10 plays a role in proximal-distal patterning. Furthermore, they show that, later in development, Fgf10 can promote the differentiation of airway epithelial cells to basal cells, a finding that has important implications for understanding and improving lung injury and repair.
Stem cell quiescence outFoxed
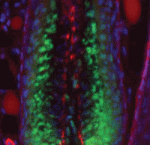 Hair follicles cyclically degenerate and regenerate through adult life: after an initial growth phase, hair follicles enter a destructive phase and then go through a quiescent stage before re-entering the next growth phase. This cycling involves hair follicle stem cells (HFSCs) but how these cells transition between the phases of the hair follicle cycle is unclear. Here, Hoang Nguyen and colleagues report that the forkhead transcription factor Foxp1 is crucial for maintaining HFSC quiescence (p. 3809). The authors show that Foxp1 is expressed in adult mouse HFSCs and that ablation of Foxp1 in skin epithelial cells shortens the quiescent phase of the hair cycle and causes precocious HFSC activation. Furthermore, they report that overexpression of Foxp1 in keratinocytes leads to cell cycle arrest as well as to upregulation of Fgf18, which has been previously implicated in controlling HFSC quiescence. Finally, the researchers demonstrate that exogenously delivered FGF18 can prevent the HFSCs of Foxp1-null mice from being prematurely activated, confirming that FGF18 acts downstream of Foxp1 to regulate stem cell quiescence.
Hair follicles cyclically degenerate and regenerate through adult life: after an initial growth phase, hair follicles enter a destructive phase and then go through a quiescent stage before re-entering the next growth phase. This cycling involves hair follicle stem cells (HFSCs) but how these cells transition between the phases of the hair follicle cycle is unclear. Here, Hoang Nguyen and colleagues report that the forkhead transcription factor Foxp1 is crucial for maintaining HFSC quiescence (p. 3809). The authors show that Foxp1 is expressed in adult mouse HFSCs and that ablation of Foxp1 in skin epithelial cells shortens the quiescent phase of the hair cycle and causes precocious HFSC activation. Furthermore, they report that overexpression of Foxp1 in keratinocytes leads to cell cycle arrest as well as to upregulation of Fgf18, which has been previously implicated in controlling HFSC quiescence. Finally, the researchers demonstrate that exogenously delivered FGF18 can prevent the HFSCs of Foxp1-null mice from being prematurely activated, confirming that FGF18 acts downstream of Foxp1 to regulate stem cell quiescence.
Fasci(cli)nating link between signal transduction and morphogenesis
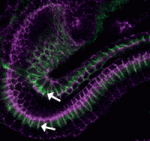 The molecular mechanisms that link intracellular signalling pathways to changes in tissue morphology are unclear. Using the Drosophila embryonic hindgut as a model, Martin Zeidler and co-workers demonstrate that the transmembrane protein Fasciclin III (FasIII) regulates intracellular adhesion and links signal transduction to morphogenesis (p. 3858). The researchers show that normal hindgut curvature is dependent on JAK/STAT signalling, and that JAK/STAT pathway activity asymmetrically localises to the inside curve of the developing hindgut, where it drives FasIII lateralisation. In addition, they demonstrate that FasIII promotes intracellular adhesion both in vivo and in cells in vitro. Based on these findings and the differential interfacial tension hypothesis, the researchers establish a mathematical model of the developing hindgut, which suggests that intracellular adhesion mediated by FasIII is sufficient to explain the curvature observed in the hindgut. These findings, together with additional studies of tissue folding in the Drosophila wing disc, suggest that FasIII-dependent modulation of intracellular adhesion might be a general mechanism by which organs are shaped during development.
The molecular mechanisms that link intracellular signalling pathways to changes in tissue morphology are unclear. Using the Drosophila embryonic hindgut as a model, Martin Zeidler and co-workers demonstrate that the transmembrane protein Fasciclin III (FasIII) regulates intracellular adhesion and links signal transduction to morphogenesis (p. 3858). The researchers show that normal hindgut curvature is dependent on JAK/STAT signalling, and that JAK/STAT pathway activity asymmetrically localises to the inside curve of the developing hindgut, where it drives FasIII lateralisation. In addition, they demonstrate that FasIII promotes intracellular adhesion both in vivo and in cells in vitro. Based on these findings and the differential interfacial tension hypothesis, the researchers establish a mathematical model of the developing hindgut, which suggests that intracellular adhesion mediated by FasIII is sufficient to explain the curvature observed in the hindgut. These findings, together with additional studies of tissue folding in the Drosophila wing disc, suggest that FasIII-dependent modulation of intracellular adhesion might be a general mechanism by which organs are shaped during development.
Dlk1 muscles out of regeneration
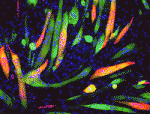 Muscle development is driven by a set of myogenic factors, but how these are regulated during normal development and during regeneration is unclear. Here (p. 3743), Charlotte Harken Jensen and colleagues show that delta-like 1 homolog (Dlk1), an imprinted gene, is a crucial regulator of the myogenic program in mice. They report that Dlk1-null mice exhibit impaired muscle development due to a defective myogenic transcriptional program: the myogenic genes Mef2c, Meis1 and Myod1 are suppressed in these mice. Surprisingly, however, they find that depletion of Dlk1, which is known to be re-expressed in regenerating muscle, in fact enhances muscle regeneration both in vitro and in vivo. This improved regenerative capacity in the absence of Dlk1 is associated with an enhanced myogenic program, and is not due to altered adipogenic-myogenic commitment. Together, these findings highlight a dual function for Dlk1 – as an enhancer of muscle development but as an inhibitor of muscle regeneration – and may open up new possibilities for improving muscle regeneration in human disease.
Muscle development is driven by a set of myogenic factors, but how these are regulated during normal development and during regeneration is unclear. Here (p. 3743), Charlotte Harken Jensen and colleagues show that delta-like 1 homolog (Dlk1), an imprinted gene, is a crucial regulator of the myogenic program in mice. They report that Dlk1-null mice exhibit impaired muscle development due to a defective myogenic transcriptional program: the myogenic genes Mef2c, Meis1 and Myod1 are suppressed in these mice. Surprisingly, however, they find that depletion of Dlk1, which is known to be re-expressed in regenerating muscle, in fact enhances muscle regeneration both in vitro and in vivo. This improved regenerative capacity in the absence of Dlk1 is associated with an enhanced myogenic program, and is not due to altered adipogenic-myogenic commitment. Together, these findings highlight a dual function for Dlk1 – as an enhancer of muscle development but as an inhibitor of muscle regeneration – and may open up new possibilities for improving muscle regeneration in human disease.
A new cloud on the horizon of mouse ooocytes
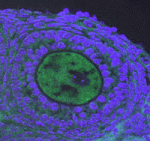 The piRNA pathway silences retrotransposons and hence maintains genome integrity in the germline. Several components of the piRNA pathway localise to a structure called the nuage, which has been detected in many animal germlines, including mouse testes and Drosophila oocytes. Now, Ai Khim Lim, Barbara Knowles and colleagues show that a nuage-like structure can be found in mouse oocytes (p. 3819). They report that the nuage proteins mouse vasa homologue (MVH), Piwi-like 2 (PIWIL2/MILI) and tudor domain-containing 9 (TDRD9) transiently colocalise to a nuage-like structure in mouse oocytes shortly after birth. Furthermore, they report, the nuage protein GASZ, which is functionally but not structurally linked to the nuage in testes, is also present in cytoplasmic granules in oocytes. Using mutant mice, the authors demonstrate that the nuage genes Mvh, Mili and Gasz control retrotransposon repression through the piRNA pathway. Importantly, however, they find that these null-mutant females, unlike their male counterparts, are fertile, thus highlighting that retrotransposon activation and sterility are uncoupled in female mice.
The piRNA pathway silences retrotransposons and hence maintains genome integrity in the germline. Several components of the piRNA pathway localise to a structure called the nuage, which has been detected in many animal germlines, including mouse testes and Drosophila oocytes. Now, Ai Khim Lim, Barbara Knowles and colleagues show that a nuage-like structure can be found in mouse oocytes (p. 3819). They report that the nuage proteins mouse vasa homologue (MVH), Piwi-like 2 (PIWIL2/MILI) and tudor domain-containing 9 (TDRD9) transiently colocalise to a nuage-like structure in mouse oocytes shortly after birth. Furthermore, they report, the nuage protein GASZ, which is functionally but not structurally linked to the nuage in testes, is also present in cytoplasmic granules in oocytes. Using mutant mice, the authors demonstrate that the nuage genes Mvh, Mili and Gasz control retrotransposon repression through the piRNA pathway. Importantly, however, they find that these null-mutant females, unlike their male counterparts, are fertile, thus highlighting that retrotransposon activation and sterility are uncoupled in female mice.
A novel role for TGFβ in lymphangiogenesis
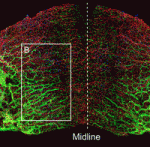 Lymphangiogenesis, the formation of lymphatic vessels, involves multiple growth factors and receptors, including vascular endothelial growth factor C (VEGFC) and its receptor VEGFR3. Here, on p. 3903, Yoh-suke Mukouyama and co-workers uncover a role for TGFβ signalling during lymphatic network development in mice. The researchers first develop a novel, whole-mount imaging technique to visualise lymphatic vessels in the anterior dorsal skin of mouse embryos. Using this approach, combined with conditional knockout of TGFβ receptors (Tgfbr1 or Tgfbr2) in lymphatic endothelial cells (LECs), they show that a loss of TGFβ signalling in LECs leads to reduced vessel sprouting and hence a global decrease in lymphatic network complexity. Furthermore, they report, LEC proliferation is increased following TGFβ receptor depletion. Finally, they demonstrate that TGFβ signalling in a dermal lymphatic cell line can upregulate the expression of VEGFR3 and the VEGFC co-receptor neuropilin 2. These studies, together with other findings, suggest that TGFβ plays a dual role during lymphangiogenesis, both enhancing LEC sprouting while decreasing LEC proliferation.
Lymphangiogenesis, the formation of lymphatic vessels, involves multiple growth factors and receptors, including vascular endothelial growth factor C (VEGFC) and its receptor VEGFR3. Here, on p. 3903, Yoh-suke Mukouyama and co-workers uncover a role for TGFβ signalling during lymphatic network development in mice. The researchers first develop a novel, whole-mount imaging technique to visualise lymphatic vessels in the anterior dorsal skin of mouse embryos. Using this approach, combined with conditional knockout of TGFβ receptors (Tgfbr1 or Tgfbr2) in lymphatic endothelial cells (LECs), they show that a loss of TGFβ signalling in LECs leads to reduced vessel sprouting and hence a global decrease in lymphatic network complexity. Furthermore, they report, LEC proliferation is increased following TGFβ receptor depletion. Finally, they demonstrate that TGFβ signalling in a dermal lymphatic cell line can upregulate the expression of VEGFR3 and the VEGFC co-receptor neuropilin 2. These studies, together with other findings, suggest that TGFβ plays a dual role during lymphangiogenesis, both enhancing LEC sprouting while decreasing LEC proliferation.
PLUS…
Cohesin in development and disease
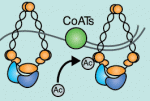 Recent studies have shown that cohesin, which was named for its ability to mediate sister chromatid cohesion, can influence gene expression during development. Here, Ana Losada and colleagues provide an overview of how cohesin functions in development and disease. See the Development at a Glance poster article on p. 3715
Recent studies have shown that cohesin, which was named for its ability to mediate sister chromatid cohesion, can influence gene expression during development. Here, Ana Losada and colleagues provide an overview of how cohesin functions in development and disease. See the Development at a Glance poster article on p. 3715
Molecular causes of aneuploidy in mammalian eggs
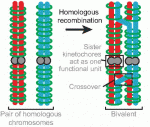 Mammalian oocytes are particularly error prone in segregating their chromosomes during their two meiotic divisions, resulting in the creation of an embryo that has inherited the wrong number of chromosomes: it is aneuploid. Here, Keith Jones and Simon Lane review recent data on factors that determine successful segregation in female meiosis and explain how this might be related to an age-related decline in female segregation accuracy. See the Primer article on p. 3719
Mammalian oocytes are particularly error prone in segregating their chromosomes during their two meiotic divisions, resulting in the creation of an embryo that has inherited the wrong number of chromosomes: it is aneuploid. Here, Keith Jones and Simon Lane review recent data on factors that determine successful segregation in female meiosis and explain how this might be related to an age-related decline in female segregation accuracy. See the Primer article on p. 3719


 (No Ratings Yet)
(No Ratings Yet)