In Development this week (Vol. 140, Issue 20)
Posted by Seema Grewal, on 1 October 2013
Here are the highlights from the new issue of Development:
Coordinating stem cell and niche development
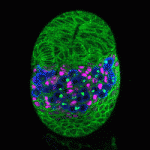 The Drosophila ovary provides an accessible system for analysing the interactions between stem cells and their supporting niche. The Insulin-like receptor (InR) and Target of rapamycin (Tor) pathways affect germline stem cell activity in the adult fly, but their roles during ovary formation are poorly understood. On p. 4145, Dana Gancz and Lilach Gilboa investigate the consequences of manipulating InR and Tor signalling in germline and gonadal somatic tissues. They find that both pathways are required cell-autonomously in primordial germ cells (PGCs) to promote their proliferation, while disrupting either pathway in the soma affects both precursor cell proliferation and niche differentiation. InR activity in somatic cells also promotes PGC differentiation non-autonomously. Importantly, these experiments reveal that the PGC-to-cyst transition is a two-step process, only the first of which is insulin regulated. Together, these data demonstrate that InR and Tor act to coordinate development of ovarian stem cells and somatic cells, helping to ensure the formation of functional niche-stem cell units.
The Drosophila ovary provides an accessible system for analysing the interactions between stem cells and their supporting niche. The Insulin-like receptor (InR) and Target of rapamycin (Tor) pathways affect germline stem cell activity in the adult fly, but their roles during ovary formation are poorly understood. On p. 4145, Dana Gancz and Lilach Gilboa investigate the consequences of manipulating InR and Tor signalling in germline and gonadal somatic tissues. They find that both pathways are required cell-autonomously in primordial germ cells (PGCs) to promote their proliferation, while disrupting either pathway in the soma affects both precursor cell proliferation and niche differentiation. InR activity in somatic cells also promotes PGC differentiation non-autonomously. Importantly, these experiments reveal that the PGC-to-cyst transition is a two-step process, only the first of which is insulin regulated. Together, these data demonstrate that InR and Tor act to coordinate development of ovarian stem cells and somatic cells, helping to ensure the formation of functional niche-stem cell units.
How to grow a heart
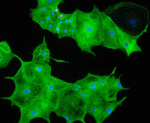 During heart development, proliferation of cardiomyocytes (CMs) must be tightly controlled to determine organ size, and different regions of the heart show differential proliferation rates. How is CM proliferation regulated in space and time, and how might the mechanisms regulating tissue growth be harnessed for clinical applications? Ibrahim Domian and co-workers investigate the role of Wnt/β-catenin signalling in regulating CM proliferation, using in vitro stem cell culture and in vivo approaches (p. 4165). They find that canonical Wnt signalling promotes proliferation of CMs derived from mouse embryonic stem cells (ESCs) or from induced pluripotent stem cells, and, importantly, of human ESC-derived CMs. Furthermore, they show that differential Wnt pathway activity likely underlies the differential proliferation rates of trabecular versus compact myocardium in the developing mouse heart in vivo. The effects of Wnt signalling on CM proliferation provides a potential method for generating large numbers of CMs in culture, which could be used for drug screening or regenerative therapy approaches.
During heart development, proliferation of cardiomyocytes (CMs) must be tightly controlled to determine organ size, and different regions of the heart show differential proliferation rates. How is CM proliferation regulated in space and time, and how might the mechanisms regulating tissue growth be harnessed for clinical applications? Ibrahim Domian and co-workers investigate the role of Wnt/β-catenin signalling in regulating CM proliferation, using in vitro stem cell culture and in vivo approaches (p. 4165). They find that canonical Wnt signalling promotes proliferation of CMs derived from mouse embryonic stem cells (ESCs) or from induced pluripotent stem cells, and, importantly, of human ESC-derived CMs. Furthermore, they show that differential Wnt pathway activity likely underlies the differential proliferation rates of trabecular versus compact myocardium in the developing mouse heart in vivo. The effects of Wnt signalling on CM proliferation provides a potential method for generating large numbers of CMs in culture, which could be used for drug screening or regenerative therapy approaches.
Chromatin regulation: KIS and tell
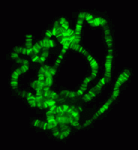 The chromatin-modifying Polycomb and trithorax group proteins are crucial for the establishment of key transcriptional networks during development. Kismet (KIS), a member of the trithorax group of proteins, promotes gene expression via transcriptional elongation as well as by antagonizing Polycomb-mediated repression; however, it is not understood how closely these two processes are linked and the molecular mechanisms that underpin them. In this issue (p. 4182), Kristel Dorighi and John Tamkun provide evidence for two distinct mechanisms of KIS-mediated gene regulation in Drosophila, separating the processes of transcriptional elongation and antagonism of Polycomb repression. The authors find that KIS recruits ASH1 to regulate levels of H3K27me3, but that ASH1 is dispensable for transcriptional elongation. Conversely, inhibition of transcriptional elongation does not affect ASH1 chromosome localization or H3K27me3 levels. Thus, the authors show that these two processes are independent and that, in addition to a general role in promoting transcriptional elongation, KIS has a separate specific role in recruiting ASH1, which regulates H3K27me3 levels and antagonizes Polycomb repression.
The chromatin-modifying Polycomb and trithorax group proteins are crucial for the establishment of key transcriptional networks during development. Kismet (KIS), a member of the trithorax group of proteins, promotes gene expression via transcriptional elongation as well as by antagonizing Polycomb-mediated repression; however, it is not understood how closely these two processes are linked and the molecular mechanisms that underpin them. In this issue (p. 4182), Kristel Dorighi and John Tamkun provide evidence for two distinct mechanisms of KIS-mediated gene regulation in Drosophila, separating the processes of transcriptional elongation and antagonism of Polycomb repression. The authors find that KIS recruits ASH1 to regulate levels of H3K27me3, but that ASH1 is dispensable for transcriptional elongation. Conversely, inhibition of transcriptional elongation does not affect ASH1 chromosome localization or H3K27me3 levels. Thus, the authors show that these two processes are independent and that, in addition to a general role in promoting transcriptional elongation, KIS has a separate specific role in recruiting ASH1, which regulates H3K27me3 levels and antagonizes Polycomb repression.
MYB in multiciliogenesis
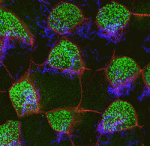 The airways of the lung are lined with multiciliated cells (MCCs), the coordinated beating of which generates fluid flow to remove particles from the lungs. Generation of MCCs requires massive amplification of centriole numbers to form the basal bodies of each cilium. How are MCCs specified and how do they differentiate? Here (p. 4277), Mark Krasnow and co-workers uncover a role for the cell cycle regulator MYB in promoting multiciliogenesis. MYB is expressed in newly postmitotic cells of the mouse airway epithelium (as well as other multiciliated cells), and mutants lacking MYB show defects in centriole amplification during MCC formation – particularly at earlier stages of development. They further show that MYB promotes the expression of the key late MCC regulator FOXJ1, while MYB itself is regulated by Notch signalling and the nuclear protein multicilin. As MYB is better known as an S-phase regulator, they propose that it may control multiciliogenesis by promoting an S-phase-like state in which centrioles are synthesized but DNA is not.
The airways of the lung are lined with multiciliated cells (MCCs), the coordinated beating of which generates fluid flow to remove particles from the lungs. Generation of MCCs requires massive amplification of centriole numbers to form the basal bodies of each cilium. How are MCCs specified and how do they differentiate? Here (p. 4277), Mark Krasnow and co-workers uncover a role for the cell cycle regulator MYB in promoting multiciliogenesis. MYB is expressed in newly postmitotic cells of the mouse airway epithelium (as well as other multiciliated cells), and mutants lacking MYB show defects in centriole amplification during MCC formation – particularly at earlier stages of development. They further show that MYB promotes the expression of the key late MCC regulator FOXJ1, while MYB itself is regulated by Notch signalling and the nuclear protein multicilin. As MYB is better known as an S-phase regulator, they propose that it may control multiciliogenesis by promoting an S-phase-like state in which centrioles are synthesized but DNA is not.
Eph-icient cell packing in the lens
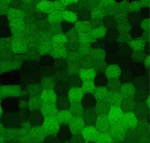 The lens of the eye is a unique tissue in that its key function, transmitting and focusing light, crucially depends on the refractive index of the tissue, and hence on the packing and alignment of lens fibre cells. Lens opacity, or cataracts, can result from defects in this precise tissue organisation. Recently, mutations in EphA2 and ephrin A5 have been associated with cataracts in both mice and humans. Here (p. 4237), Xiaohua Gong and colleagues analyse the mechanisms by which Eph/ephrin signalling regulates lens cell organisation in mice. The authors find that EphA2 regulates the subcellular localisation of adhesion and cytoskeletal proteins such as cortactin, F-actin and E-cadherin. These effects are likely mediated – at least in part – by Src kinase, the localisation of which is also dependent upon EphA2. Cell shape and packing are disrupted in Epha2 and Src mutants, suggesting that Eph/ephrin signalling via Src plays a key role in regulating cytoskeletal organisation in lens fibre cells and their arrangement in the tissue.
The lens of the eye is a unique tissue in that its key function, transmitting and focusing light, crucially depends on the refractive index of the tissue, and hence on the packing and alignment of lens fibre cells. Lens opacity, or cataracts, can result from defects in this precise tissue organisation. Recently, mutations in EphA2 and ephrin A5 have been associated with cataracts in both mice and humans. Here (p. 4237), Xiaohua Gong and colleagues analyse the mechanisms by which Eph/ephrin signalling regulates lens cell organisation in mice. The authors find that EphA2 regulates the subcellular localisation of adhesion and cytoskeletal proteins such as cortactin, F-actin and E-cadherin. These effects are likely mediated – at least in part – by Src kinase, the localisation of which is also dependent upon EphA2. Cell shape and packing are disrupted in Epha2 and Src mutants, suggesting that Eph/ephrin signalling via Src plays a key role in regulating cytoskeletal organisation in lens fibre cells and their arrangement in the tissue.
APC: Assuring Proper Centrosome separation
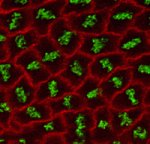 The adenomatous polyposis coli (APC) tumour suppressor is best known as a Wnt signalling regulator, but it also plays cytoskeletal roles, including during mitosis. APC mutant cells show enhanced chromosomal instability, while in early Drosophila embryos undergoing syncytial mitoses, APC2 mutants show enhanced ‘nuclear fallout’ – whereby nuclei that have undergone defective division are removed from the cortex. To understand the mechanisms underlying the mitotic function of APC, Mark Peifer and colleagues (p. 4226) have analysed the mitotic defects in APC2 mutant Drosophila embryos in detail. Their data suggest that APC2 is not essential for mitosis, but does promote mitotic fidelity. The primary consequence of APC2 loss appears to be a defect in centrosome separation; when this fails, the mitotic spindle can still form, but ectopic cleavage furrows appear and disrupt chromosome segregation. Moreover, the APC-binding partner Axin is also involved in this process. These data suggest a key role for APC family proteins in promoting centrosome separation during mitosis; this may also be relevant for its tumour suppressor activity.
The adenomatous polyposis coli (APC) tumour suppressor is best known as a Wnt signalling regulator, but it also plays cytoskeletal roles, including during mitosis. APC mutant cells show enhanced chromosomal instability, while in early Drosophila embryos undergoing syncytial mitoses, APC2 mutants show enhanced ‘nuclear fallout’ – whereby nuclei that have undergone defective division are removed from the cortex. To understand the mechanisms underlying the mitotic function of APC, Mark Peifer and colleagues (p. 4226) have analysed the mitotic defects in APC2 mutant Drosophila embryos in detail. Their data suggest that APC2 is not essential for mitosis, but does promote mitotic fidelity. The primary consequence of APC2 loss appears to be a defect in centrosome separation; when this fails, the mitotic spindle can still form, but ectopic cleavage furrows appear and disrupt chromosome segregation. Moreover, the APC-binding partner Axin is also involved in this process. These data suggest a key role for APC family proteins in promoting centrosome separation during mitosis; this may also be relevant for its tumour suppressor activity.
PLUS…
Sox proteins: regulators of cell fate specification and differentiation
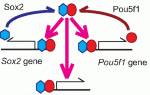 Sox transcription factors play widespread roles during development. Here, Kamachi and Kondoh provide an mechanistic overview of how Sox family proteins function and how they can thus regulate diverse processes during development. See the Primer on p. 4129
Sox transcription factors play widespread roles during development. Here, Kamachi and Kondoh provide an mechanistic overview of how Sox family proteins function and how they can thus regulate diverse processes during development. See the Primer on p. 4129
Studies of morphogens: keep calm and carry on
 The recent EMBO Workshop on ‘Morphogen gradients’, which took place in Oxford, UK in June 2013, centered on the formation and interpretation of morphogen gradients during development. Here, Angelike Stathopoulos and Dagmar Iber provide a brief overview of the talks and discussion at the meeting. See the Meeting Review on p. 4119
The recent EMBO Workshop on ‘Morphogen gradients’, which took place in Oxford, UK in June 2013, centered on the formation and interpretation of morphogen gradients during development. Here, Angelike Stathopoulos and Dagmar Iber provide a brief overview of the talks and discussion at the meeting. See the Meeting Review on p. 4119
Getting the measure of things: the physical biology of stem cells
 In July 2013, the diverse fields of biology, physics and mathematics converged to discuss ‘The Physical Biology of Stem Cells’, the subject of the third annual symposium of the Cambridge Stem Cell Institute, UK. Here, Sally Lowell reviews the meeting and the themes that emerged from it. See the Meeting Review on p. 4125
In July 2013, the diverse fields of biology, physics and mathematics converged to discuss ‘The Physical Biology of Stem Cells’, the subject of the third annual symposium of the Cambridge Stem Cell Institute, UK. Here, Sally Lowell reviews the meeting and the themes that emerged from it. See the Meeting Review on p. 4125


 (No Ratings Yet)
(No Ratings Yet)