In Development this week (Vol. 140, Issue 21)
Posted by Seema Grewal, on 15 October 2013
Here are the highlights from the current issue of Development:
Deconstructing pancreas development in vitro
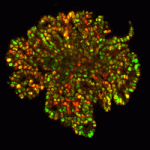 An effective cellular therapy for diabetes is dependent on the production of sufficient quantities of functional β-cells. Recent studies have enabled the production of pancreatic precursors but efforts to expand these cells and differentiate them into insulin-producing β-cells have proved a challenge. Now, Anne Grapin-Botton and colleagues establish a three-dimensional culture method that enables the efficient expansion of mouse embryonic pancreatic progenitors (p. 4452). They show that, when cultured at low density in Matrigel, dissociated epithelial cells from E10.5 mouse pancreata proliferate and form branched pancreatic organoids. These organoids, they report, consist of polarised epithelial cells lining a lumen, with some cells expressing endocrine markers. The authors further show that organoid formation is dependent on a community effect; a minimum of four pancreatic cells in the initial cluster is required for subsequent organoid development. Using the culture system, the authors also dissect several other aspects of pancreas development, highlighting that this culture method offers huge long-term potential in uncovering important aspects of β-cell development.
An effective cellular therapy for diabetes is dependent on the production of sufficient quantities of functional β-cells. Recent studies have enabled the production of pancreatic precursors but efforts to expand these cells and differentiate them into insulin-producing β-cells have proved a challenge. Now, Anne Grapin-Botton and colleagues establish a three-dimensional culture method that enables the efficient expansion of mouse embryonic pancreatic progenitors (p. 4452). They show that, when cultured at low density in Matrigel, dissociated epithelial cells from E10.5 mouse pancreata proliferate and form branched pancreatic organoids. These organoids, they report, consist of polarised epithelial cells lining a lumen, with some cells expressing endocrine markers. The authors further show that organoid formation is dependent on a community effect; a minimum of four pancreatic cells in the initial cluster is required for subsequent organoid development. Using the culture system, the authors also dissect several other aspects of pancreas development, highlighting that this culture method offers huge long-term potential in uncovering important aspects of β-cell development.
See the press release from DanStem here
aPKC sorts out blastocyst formation
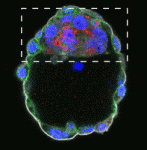 The preimplantation mouse embryo consists of three lineages: trophectoderm, primitive endoderm (PrE) and epiblast (Epi), which will become the future foetus. PrE and Epi precursors are initially present in a ‘salt and pepper’ distribution within the blastocyst and subsequently sort into two distinct layers but what controls this segregation? Here, Berenika Plusa and colleagues show that atypical protein kinase C (aPKC) couples cell sorting with cell fate progression in the mouse blastocyst (p. 4311). They first show that aPKC is enriched in PrE precursors prior to cell sorting. This enrichment, they report, is dependent on FGF signalling and the acquisition of PrE fate. Importantly, RNAi knockdown or chemical inhibition of aPKC impairs PrE-Epi segregation. Finally, the authors demonstrate that inhibition of aPKC also compromises the maturation of PrE cells; embryos treated with aPKC inhibitor fail to form a PrE layer and concomitantly fail to develop a polarised apical surface. The authors thus propose that aPKC links cell sorting with the progression of cell differentiation in the blastocyst.
The preimplantation mouse embryo consists of three lineages: trophectoderm, primitive endoderm (PrE) and epiblast (Epi), which will become the future foetus. PrE and Epi precursors are initially present in a ‘salt and pepper’ distribution within the blastocyst and subsequently sort into two distinct layers but what controls this segregation? Here, Berenika Plusa and colleagues show that atypical protein kinase C (aPKC) couples cell sorting with cell fate progression in the mouse blastocyst (p. 4311). They first show that aPKC is enriched in PrE precursors prior to cell sorting. This enrichment, they report, is dependent on FGF signalling and the acquisition of PrE fate. Importantly, RNAi knockdown or chemical inhibition of aPKC impairs PrE-Epi segregation. Finally, the authors demonstrate that inhibition of aPKC also compromises the maturation of PrE cells; embryos treated with aPKC inhibitor fail to form a PrE layer and concomitantly fail to develop a polarised apical surface. The authors thus propose that aPKC links cell sorting with the progression of cell differentiation in the blastocyst.
Hoxa2 is all ears
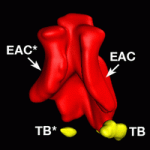 Abnormalities in the external ear, which is composed of the auricle and the external auditory canal (EAC), are frequent in newborns. However, little is known about the molecular mechanisms that govern external ear morphogenesis. Here, Filippo Rijli and colleagues report that Hoxa2 is a key transcriptional regulator that controls auricle morphogenesis in mice (p. 4386). The researchers show that the mouse auricle derives from Hoxa2-expressing neural crest mesenchyme of the second pharyngeal arch, and not from the first and second arches as previously proposed. Furthermore, they report, the lining of the EAC derives from Hoxa2-negative first arch mesenchyme. Their analysis of gene expression patterns in wild-type and Hoxa2 mutant mice suggests that Hoxa2 organises patterns of cell proliferation during ear morphogenesis, acting in part through BMP signalling. Finally, they find that ectopic expression of Hoxa2 in the neural crest of the first arch results in auricle duplication, suggesting that Hoxa2 is able to induce and maintain the molecular programme that underlies auricle formation.
Abnormalities in the external ear, which is composed of the auricle and the external auditory canal (EAC), are frequent in newborns. However, little is known about the molecular mechanisms that govern external ear morphogenesis. Here, Filippo Rijli and colleagues report that Hoxa2 is a key transcriptional regulator that controls auricle morphogenesis in mice (p. 4386). The researchers show that the mouse auricle derives from Hoxa2-expressing neural crest mesenchyme of the second pharyngeal arch, and not from the first and second arches as previously proposed. Furthermore, they report, the lining of the EAC derives from Hoxa2-negative first arch mesenchyme. Their analysis of gene expression patterns in wild-type and Hoxa2 mutant mice suggests that Hoxa2 organises patterns of cell proliferation during ear morphogenesis, acting in part through BMP signalling. Finally, they find that ectopic expression of Hoxa2 in the neural crest of the first arch results in auricle duplication, suggesting that Hoxa2 is able to induce and maintain the molecular programme that underlies auricle formation.
An eye for switching cell fate
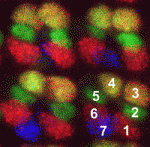 Cell fate decisions are influenced by extrinsic and intrinsic factors, but understanding how these are integrated is key for determining how cell fate is specified during development. Here, Yannis Mavromatakis and Andrew Tomlinson use developing ommatidia of the Drosophila eye as a model for exploring the logic of cell fate decisions (p. 4353). The ommatidia are constructed in two distinct waves. It is known that the transcription factor Lozenge (Lz) is expressed in second wave cells and that Notch/receptor tyrosine kinase (N/RTK) signalling is involved in specifying each of the cell fates generated in the second wave. In this study, the researchers ectopically express Lz in first wave cells, supply them with appropriate N/RTK codes, and thereby reproduce each of the second wave cell fates. Based on the dissection of this series of experiments, they conclude that Lz provides key intrinsic information to second wave cells. They also infer that N/RTK activities, in concert with Lz, are only required for a short period of time to ‘lock in’ cell fate.
Cell fate decisions are influenced by extrinsic and intrinsic factors, but understanding how these are integrated is key for determining how cell fate is specified during development. Here, Yannis Mavromatakis and Andrew Tomlinson use developing ommatidia of the Drosophila eye as a model for exploring the logic of cell fate decisions (p. 4353). The ommatidia are constructed in two distinct waves. It is known that the transcription factor Lozenge (Lz) is expressed in second wave cells and that Notch/receptor tyrosine kinase (N/RTK) signalling is involved in specifying each of the cell fates generated in the second wave. In this study, the researchers ectopically express Lz in first wave cells, supply them with appropriate N/RTK codes, and thereby reproduce each of the second wave cell fates. Based on the dissection of this series of experiments, they conclude that Lz provides key intrinsic information to second wave cells. They also infer that N/RTK activities, in concert with Lz, are only required for a short period of time to ‘lock in’ cell fate.
Vascular biomechanics in full flow
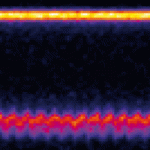 Pulsatile blood flow is driven by the heart and is a universal feature of vertebrate blood systems. However, the mechanisms controlling blood flow propagation in the embryo, while heart maturation is ongoing, are poorly understood. Here, Julien Vermot and co-workers examine vascular hydrodynamics and biomechanics in zebrafish embryos (p. 4426). Using high temporal resolution imaging together with an optical tweezer-based approach, the authors characterise the flow within the embryonic vascular network. They show that strong flow rectification occurs between branches of the network, suggesting that an additional force is generated within the network. Based on the observed movement of blood cells within the embryonic artery, the authors postulate that elasticity of the network is essential for mediating this effect. Following this, they develop a mathematical model of flow within the network and propose that the dorsal aorta acts as a capacitor that inflates and deflates in response to heartbeats. They propose that this capacitive mechanism has a major role in setting early flow propagation and reducing embryonic heart effort.
Pulsatile blood flow is driven by the heart and is a universal feature of vertebrate blood systems. However, the mechanisms controlling blood flow propagation in the embryo, while heart maturation is ongoing, are poorly understood. Here, Julien Vermot and co-workers examine vascular hydrodynamics and biomechanics in zebrafish embryos (p. 4426). Using high temporal resolution imaging together with an optical tweezer-based approach, the authors characterise the flow within the embryonic vascular network. They show that strong flow rectification occurs between branches of the network, suggesting that an additional force is generated within the network. Based on the observed movement of blood cells within the embryonic artery, the authors postulate that elasticity of the network is essential for mediating this effect. Following this, they develop a mathematical model of flow within the network and propose that the dorsal aorta acts as a capacitor that inflates and deflates in response to heartbeats. They propose that this capacitive mechanism has a major role in setting early flow propagation and reducing embryonic heart effort.
A role for GPCRs in neurogenesis
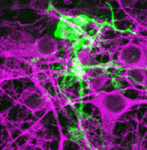 During development of the mammalian brain, cortical progenitors divide and give rise to neurons and glia. A number of signalling molecules and receptors are known to control neural progenitor fate but now (see p. 4335) Kamon Sanada and co-workers uncover a role for G protein-coupled receptor (GPCR) signalling during neurogenesis in the developing mouse neocortex. The authors demonstrate that GPRC5B, an orphan GPCR, is expressed in cortical progenitors of the developing mouse brain. Using RNAi-mediated knockdown, they report that GPRC5B is required for neuronal differentiation; GPRC5B-depleted progenitors fail to become neurons and instead adopt an astrocyte fate. The researchers further show that GPRC5B couples with the G12/13 class of heterotrimeric G proteins in cultured cells, and that GPRC5B signalling may converge with β-catenin signalling both in vitro and in vivo. In summary, these studies uncover a novel and important role for GPCR signalling during cortical neurogenesis, a finding that has significant implications for the therapeutic targeting and manipulation of stem cells.
During development of the mammalian brain, cortical progenitors divide and give rise to neurons and glia. A number of signalling molecules and receptors are known to control neural progenitor fate but now (see p. 4335) Kamon Sanada and co-workers uncover a role for G protein-coupled receptor (GPCR) signalling during neurogenesis in the developing mouse neocortex. The authors demonstrate that GPRC5B, an orphan GPCR, is expressed in cortical progenitors of the developing mouse brain. Using RNAi-mediated knockdown, they report that GPRC5B is required for neuronal differentiation; GPRC5B-depleted progenitors fail to become neurons and instead adopt an astrocyte fate. The researchers further show that GPRC5B couples with the G12/13 class of heterotrimeric G proteins in cultured cells, and that GPRC5B signalling may converge with β-catenin signalling both in vitro and in vivo. In summary, these studies uncover a novel and important role for GPCR signalling during cortical neurogenesis, a finding that has significant implications for the therapeutic targeting and manipulation of stem cells.
PLUS…
To branch or not to branch: the role of pre-patterning in lateral root formation
 The establishment of a pre-pattern or competence to form new organs is a key feature of plant development. Philip Benfey, Tom Beeckman and colleagues review the mechanisms that underlie pre-pattern formation and the earliest stages of lateral root development. See the Review article on p. 4301
The establishment of a pre-pattern or competence to form new organs is a key feature of plant development. Philip Benfey, Tom Beeckman and colleagues review the mechanisms that underlie pre-pattern formation and the earliest stages of lateral root development. See the Review article on p. 4301
An interview with Janet Rossant
 Janet Rossant is a developmental biologist who has worked for many years on the mouse blastocyst, the derivation of stem cell lines and on investigating the mouse genome. In June 2013 she became president of the ISSCR, and she was also awarded the Harrison Medal – a prestigious prize given only once every four years. Earlier this year, Cat Vicente interviewed Janet. See the Spotlight article on p. 4299
Janet Rossant is a developmental biologist who has worked for many years on the mouse blastocyst, the derivation of stem cell lines and on investigating the mouse genome. In June 2013 she became president of the ISSCR, and she was also awarded the Harrison Medal – a prestigious prize given only once every four years. Earlier this year, Cat Vicente interviewed Janet. See the Spotlight article on p. 4299


 (No Ratings Yet)
(No Ratings Yet)