In Development this week (Vol. 140, Issue 23)
Posted by Seema Grewal, on 19 November 2013
Here are the highlights from the new issue of Development:
Broken-hearted over Hippo
 Mammalian cardiac regeneration is greatly impeded by the massive loss of cardiomyocytes that occurs following acute injury. The failure of the remaining cells to proliferate is a considerable challenge for the field, but the molecular mechanisms that control cardiomyocyte proliferation in the adult heart are largely unknown. Now, on p. 4683, James Martin and colleagues demonstrate a role for Hippo signalling in suppressing adult and postnatal murine cardiomyocyte proliferation. Using conditional knockouts, the authors show that removal of Hippo pathway members Salv or Lats1 and Lats2 from normal adult cardiomyocytes results in increased proliferation, as these cells are able to re-enter the cell cycle and undergo cytokinesis. Moreover, removal of Salv from cardiomyocytes in vivo results in improved cardiac regeneration after adult myocardial infarction, a time when regeneration is usually severely impaired. Here, the authors observed reduced scarring and full restoration of cardiac function. This elegant study suggests that Hippo signalling is a repressor of adult cardiomyocyte renewal and regeneration.
Mammalian cardiac regeneration is greatly impeded by the massive loss of cardiomyocytes that occurs following acute injury. The failure of the remaining cells to proliferate is a considerable challenge for the field, but the molecular mechanisms that control cardiomyocyte proliferation in the adult heart are largely unknown. Now, on p. 4683, James Martin and colleagues demonstrate a role for Hippo signalling in suppressing adult and postnatal murine cardiomyocyte proliferation. Using conditional knockouts, the authors show that removal of Hippo pathway members Salv or Lats1 and Lats2 from normal adult cardiomyocytes results in increased proliferation, as these cells are able to re-enter the cell cycle and undergo cytokinesis. Moreover, removal of Salv from cardiomyocytes in vivo results in improved cardiac regeneration after adult myocardial infarction, a time when regeneration is usually severely impaired. Here, the authors observed reduced scarring and full restoration of cardiac function. This elegant study suggests that Hippo signalling is a repressor of adult cardiomyocyte renewal and regeneration.
Osr2 PAX a punch in palate formation
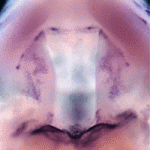 Precise orchestration of palate formation involves the complex interaction of signalling cascades and transcriptional networks in the developing craniofacial region. Pax9 and Osr2 have previously been implicated in palate formation, but little is known about how these molecular components interact within the greater regulatory network. Now, on p. 4709, Rulang Jiang and colleagues report a crucial role for Pax9 in patterning the anterior-posterior axis as well as outgrowth of the developing palatal shelves. The authors show that Pax9 regulates mesenchyme-epithelium interactions during pattern formation and that the expression of several key genes involved in palate development, such as Shh, Bmp4, Fgf10, Msx1 and Osr2, is reduced in Pax9 mutant mice. Interestingly, expression of Osr2 from the Pax9 locus was able to rescue the posterior, but not anterior, palate formation defect in the absence of Pax9 function. These data place Pax9 upstream of transcription factor Osr2 and signalling molecules Bmp4, Fgf10 and Shh in the molecular network that regulates palate development.
Precise orchestration of palate formation involves the complex interaction of signalling cascades and transcriptional networks in the developing craniofacial region. Pax9 and Osr2 have previously been implicated in palate formation, but little is known about how these molecular components interact within the greater regulatory network. Now, on p. 4709, Rulang Jiang and colleagues report a crucial role for Pax9 in patterning the anterior-posterior axis as well as outgrowth of the developing palatal shelves. The authors show that Pax9 regulates mesenchyme-epithelium interactions during pattern formation and that the expression of several key genes involved in palate development, such as Shh, Bmp4, Fgf10, Msx1 and Osr2, is reduced in Pax9 mutant mice. Interestingly, expression of Osr2 from the Pax9 locus was able to rescue the posterior, but not anterior, palate formation defect in the absence of Pax9 function. These data place Pax9 upstream of transcription factor Osr2 and signalling molecules Bmp4, Fgf10 and Shh in the molecular network that regulates palate development.
Par3 makes contact in migrating mesenchyme
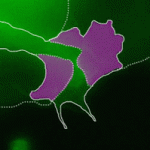 Contact inhibition of locomotion (CIL) is a fundamental regulatory mechanism that ensures correct cell movement and migration. During CIL, cells form transient contacts but the molecular nature of such contacts is unknown. In this issue, Roberto Mayor and colleagues (p. 4763) investigate the role of the cell polarity protein Par3 in microtubule collapse and reorganisation during CIL in migrating neural crest cells. Using antisense morpholinos to Par3 in Xenopus and zebrafish, the authors show that loss of Par3 has a dramatic effect on migration and is essential for CIL both in vitro and in vivo. Par3 knockdowns fail to exhibit microtubule collapse at the cell-cell contact; however, this can be rescued by injection of an antisense morpholino to Trio, implicating the Rac-GEF Trio in migrating neural crest CIL. The authors propose a model in which CIL requires the local destabilisation of microtubules at the cell-cell contacts, which is controlled in a Par3/Trio-dependent manner.
Contact inhibition of locomotion (CIL) is a fundamental regulatory mechanism that ensures correct cell movement and migration. During CIL, cells form transient contacts but the molecular nature of such contacts is unknown. In this issue, Roberto Mayor and colleagues (p. 4763) investigate the role of the cell polarity protein Par3 in microtubule collapse and reorganisation during CIL in migrating neural crest cells. Using antisense morpholinos to Par3 in Xenopus and zebrafish, the authors show that loss of Par3 has a dramatic effect on migration and is essential for CIL both in vitro and in vivo. Par3 knockdowns fail to exhibit microtubule collapse at the cell-cell contact; however, this can be rescued by injection of an antisense morpholino to Trio, implicating the Rac-GEF Trio in migrating neural crest CIL. The authors propose a model in which CIL requires the local destabilisation of microtubules at the cell-cell contacts, which is controlled in a Par3/Trio-dependent manner.
Designer flies: accelerated genome editing in Drosophila
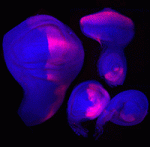 The immense power of Drosophila genetics has allowed invaluable insight into developmental biology. Despite these advances, a significant limitation has always been the lack of an efficient method for modifying select genetic loci. Now, on p. 4818, Jean-Paul Vincent and colleagues report high-efficiency homologous recombination in Drosophila with a novel gene-targeting vector. This can be achieved via a two-generation crossing scheme or via direct embryo injection. Importantly, both approaches yield few false-positives due to efficient negative selection, while readily detectable markers aid in the rapid identification of correctly targeted flies. The efficiency can be further increased by co-injecting the sequence-specific endonuclease CRISPR/Cas9. The investigators also report a series of vectors that can be used to insert different genetic elements into the targeted loci, such as mutated or tagged cDNAs and additional reporter genes. Their approach will enable genetic modification in a wide range of contexts, including in postmitotic cells. These tools will be a valuable resource for the Drosophila community.
The immense power of Drosophila genetics has allowed invaluable insight into developmental biology. Despite these advances, a significant limitation has always been the lack of an efficient method for modifying select genetic loci. Now, on p. 4818, Jean-Paul Vincent and colleagues report high-efficiency homologous recombination in Drosophila with a novel gene-targeting vector. This can be achieved via a two-generation crossing scheme or via direct embryo injection. Importantly, both approaches yield few false-positives due to efficient negative selection, while readily detectable markers aid in the rapid identification of correctly targeted flies. The efficiency can be further increased by co-injecting the sequence-specific endonuclease CRISPR/Cas9. The investigators also report a series of vectors that can be used to insert different genetic elements into the targeted loci, such as mutated or tagged cDNAs and additional reporter genes. Their approach will enable genetic modification in a wide range of contexts, including in postmitotic cells. These tools will be a valuable resource for the Drosophila community.
Puffyeye regulates Myc-mediated cell growth
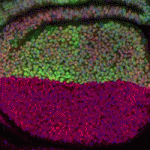 Proper control of cell size is vital to ensure the correct growth and development of any organism. The Myc family of proteins are key regulators of growth, but the mechanisms that control Myc protein levels are complex. Now, on p. 4776, Robert Eisenman and colleagues identify Drosophila Puffyeye (Puf), an orthologue of mammalian USP34, as a novel ubiquitin-specific protease (USP) regulating dMyc-dependant cell growth at the post-translational level. Using genetic interaction experiments, the authors demonstrate that puf opposes the activity of the ubiquitin ligase archipelago (ago) and that Puf acts to stabilise dMyc protein levels. Overexpression of puf in the eye and wing phenocopies dMyc overexpression, while expression of a catalytically inactive form of Puf had no effect, demonstrating the requirement of the Puf USP catalytic domain. Interestingly, the authors demonstrated that Puf can also regulate Ago and Cyclin E protein levels. These data reveal a new mechanism by which dMyc levels can be regulated by USPs in order to fine-tune cell growth.
Proper control of cell size is vital to ensure the correct growth and development of any organism. The Myc family of proteins are key regulators of growth, but the mechanisms that control Myc protein levels are complex. Now, on p. 4776, Robert Eisenman and colleagues identify Drosophila Puffyeye (Puf), an orthologue of mammalian USP34, as a novel ubiquitin-specific protease (USP) regulating dMyc-dependant cell growth at the post-translational level. Using genetic interaction experiments, the authors demonstrate that puf opposes the activity of the ubiquitin ligase archipelago (ago) and that Puf acts to stabilise dMyc protein levels. Overexpression of puf in the eye and wing phenocopies dMyc overexpression, while expression of a catalytically inactive form of Puf had no effect, demonstrating the requirement of the Puf USP catalytic domain. Interestingly, the authors demonstrated that Puf can also regulate Ago and Cyclin E protein levels. These data reveal a new mechanism by which dMyc levels can be regulated by USPs in order to fine-tune cell growth.
GDF5 determines dendrite growth
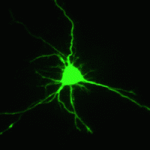 Dendrite complexity determines the functional properties of neurons and the overall connectivity of neuronal circuits. The bone morphogenetic protein (BMP) family is known to regulate a myriad of developmental processes, but the extent to which different members of the family are involved in dendrite growth remains unclear. In this issue (p. 4751), Alun Davies and colleagues identify growth differentiation factor 5 (GDF5), a member of the BMP family, as a key regulator of dendrite growth and complexity in the pyramidal neurons of the developing hippocampus. Mice harbouring a mutation in Gdf5 showed dramatically reduced dendrite size and complexity. In vitro, exogenous GDF5 treatment was sufficient to increase elongation of the dendrites, but not the axons, of pyramidal cells derived from the developing mouse hippocampus. The authors further demonstrated that GDF5-mediated dendrite growth acts via the Smad signalling pathway and that GDF5-regulated HES5 expression is both necessary and sufficient for enhanced dendritic growth and complexity.
Dendrite complexity determines the functional properties of neurons and the overall connectivity of neuronal circuits. The bone morphogenetic protein (BMP) family is known to regulate a myriad of developmental processes, but the extent to which different members of the family are involved in dendrite growth remains unclear. In this issue (p. 4751), Alun Davies and colleagues identify growth differentiation factor 5 (GDF5), a member of the BMP family, as a key regulator of dendrite growth and complexity in the pyramidal neurons of the developing hippocampus. Mice harbouring a mutation in Gdf5 showed dramatically reduced dendrite size and complexity. In vitro, exogenous GDF5 treatment was sufficient to increase elongation of the dendrites, but not the axons, of pyramidal cells derived from the developing mouse hippocampus. The authors further demonstrated that GDF5-mediated dendrite growth acts via the Smad signalling pathway and that GDF5-regulated HES5 expression is both necessary and sufficient for enhanced dendritic growth and complexity.
PLUS…
Nutritional regulation of stem and progenitor cells in Drosophila
 Stem cells and their progenitors are maintained within a microenvironment, termed the niche, but it is known that systemic signals originating outside the niche also affect stem cell and progenitor behavior. Here, Utpal Banerjee and colleagues review recent studies of nutritional effects on stem and progenitor cell maintenance and proliferation in Drosophila. See the Review article on p. 4647
Stem cells and their progenitors are maintained within a microenvironment, termed the niche, but it is known that systemic signals originating outside the niche also affect stem cell and progenitor behavior. Here, Utpal Banerjee and colleagues review recent studies of nutritional effects on stem and progenitor cell maintenance and proliferation in Drosophila. See the Review article on p. 4647
Cell-intrinsic drivers of dendrite morphogenesis
 The proper formation and morphogenesis of dendrites is fundamental to the establishment of neural circuits in the brain. In this issue, Sidharth Puram and Azad Bonni review cell-intrinsic drivers of dendrite patterning and discuss how the characterization of such regulators advances our understanding of normal brain development and pathogenesis of diverse cognitive disorders. See the Review on p. 4657
The proper formation and morphogenesis of dendrites is fundamental to the establishment of neural circuits in the brain. In this issue, Sidharth Puram and Azad Bonni review cell-intrinsic drivers of dendrite patterning and discuss how the characterization of such regulators advances our understanding of normal brain development and pathogenesis of diverse cognitive disorders. See the Review on p. 4657


 (No Ratings Yet)
(No Ratings Yet)