In Development this week (Vol. 141, Issue 3)
Posted by Seema Grewal, on 21 January 2014
Here are the highlights from the current issue of Development:
Hoxb1b gets the neural tube into shape
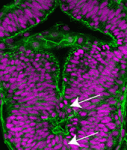 Hox genes are classically known for their roles in patterning the anterior-posterior axis of animals. Now, on p. 639, Mihaela Žigman, Cecilia Moens and colleagues uncover a new role for Hoxb1b in regulating cell shape, oriented divisions and microtubule dynamics in the developing zebrafish neural tube. The researchers first identify a zebrafish mutant that carries a point mutation in hoxb1b, a gene that shares ancestral functions with mammalian Hoxa1. These mutants, they report, exhibit classical homeotic transformations associated with Hoxa1 mutations in mice. Unexpectedly, however, these mutants also show defective neuroepithelial morphogenesis within the developing hindbrain neural tube. The researchers further show that the hoxb1b mutation does not affect apico-basal or adherens junction-based polarisation, nor the proliferation or differentiation rate of neural progenitors. Instead, Hoxb1b regulates mitotic spindle orientation and the shape of progenitor cells. This function is linked to a cell-non-autonomous role for Hoxb1b in regulating microtubule dynamics. The authors thus propose that, by regulating microtubule dynamics and cell shape, Hox genes can influence global tissue morphogenetic events.
Hox genes are classically known for their roles in patterning the anterior-posterior axis of animals. Now, on p. 639, Mihaela Žigman, Cecilia Moens and colleagues uncover a new role for Hoxb1b in regulating cell shape, oriented divisions and microtubule dynamics in the developing zebrafish neural tube. The researchers first identify a zebrafish mutant that carries a point mutation in hoxb1b, a gene that shares ancestral functions with mammalian Hoxa1. These mutants, they report, exhibit classical homeotic transformations associated with Hoxa1 mutations in mice. Unexpectedly, however, these mutants also show defective neuroepithelial morphogenesis within the developing hindbrain neural tube. The researchers further show that the hoxb1b mutation does not affect apico-basal or adherens junction-based polarisation, nor the proliferation or differentiation rate of neural progenitors. Instead, Hoxb1b regulates mitotic spindle orientation and the shape of progenitor cells. This function is linked to a cell-non-autonomous role for Hoxb1b in regulating microtubule dynamics. The authors thus propose that, by regulating microtubule dynamics and cell shape, Hox genes can influence global tissue morphogenetic events.
A new model for bivalency
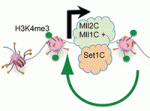 Histone H3 lysine 4 trimethylation (H3K4me3) is a universal epigenetic mark that is deposited by histone methyltransferases. This mark can be found in the context of bivalent promoters, which harbour both repressive H3K4me3 and active H3K27me3 marks and hence are thought to be poised for lineage-specific activation or repression. Here, Francis Stewart, Henk Stunnenberg and co-workers challenge this model of poising (p. 526). They first show that the H3K4 methyltransferase Mll2 is responsible for H3K4me3 on bivalent promoters in embryonic stem cells (ESCs). Accordingly, the researchers find that Mll2 is bound to bivalent promoters but also to active promoters. By contrast, another H3K4 methyltransferase, Set1C, is bound to active but not bivalent promoters. Importantly, they observe that Mll2-deficent ESCs, which lack H3K4me3 on bivalent promoters, exhibit normal transcription responsiveness, thus arguing against a model of poising. Based on these and other findings, the authors propose that Mll2 acts as a pioneer methyltransferase and that Polygroup group action on bivalent promoters blocks the establishment of active Set1C-bound promoters.
Histone H3 lysine 4 trimethylation (H3K4me3) is a universal epigenetic mark that is deposited by histone methyltransferases. This mark can be found in the context of bivalent promoters, which harbour both repressive H3K4me3 and active H3K27me3 marks and hence are thought to be poised for lineage-specific activation or repression. Here, Francis Stewart, Henk Stunnenberg and co-workers challenge this model of poising (p. 526). They first show that the H3K4 methyltransferase Mll2 is responsible for H3K4me3 on bivalent promoters in embryonic stem cells (ESCs). Accordingly, the researchers find that Mll2 is bound to bivalent promoters but also to active promoters. By contrast, another H3K4 methyltransferase, Set1C, is bound to active but not bivalent promoters. Importantly, they observe that Mll2-deficent ESCs, which lack H3K4me3 on bivalent promoters, exhibit normal transcription responsiveness, thus arguing against a model of poising. Based on these and other findings, the authors propose that Mll2 acts as a pioneer methyltransferase and that Polygroup group action on bivalent promoters blocks the establishment of active Set1C-bound promoters.
InSpired dendrite architecture
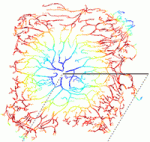 The correct architecture of dendritic trees is essential for the wiring and function of neuronal circuits. A number of cell extrinsic factors are known to regulate dendrite shape and patterning, but here Don van Meyel and co-workers show that the transcription factor Longitudinals Lacking (Lola) regulates expression of the actin nucleation protein Spire (Spir) to sculpt dendrite architecture in Drosophila (p. 650). The researchers show that Lola is expressed in dendritic arborisation (da) neurons of the Drosophila peripheral nervous system. They further demonstrate that Lola controls the number, growth and distribution of dendrite branches in da neurons. Loss of Lola also leads to increased expression of Spir, which in turn causes increased formation of abnormal and inappropriately positioned actin-rich branches. In line with this, the authors report that Spir promotes F-actin nucleation and regulates dendrite positioning. Together, these findings suggest that Lola acts to limit the expression of Spir within da neurons, thus ensuring balanced control of the actin cytoskeleton and regulated dendrite morphogenesis.
The correct architecture of dendritic trees is essential for the wiring and function of neuronal circuits. A number of cell extrinsic factors are known to regulate dendrite shape and patterning, but here Don van Meyel and co-workers show that the transcription factor Longitudinals Lacking (Lola) regulates expression of the actin nucleation protein Spire (Spir) to sculpt dendrite architecture in Drosophila (p. 650). The researchers show that Lola is expressed in dendritic arborisation (da) neurons of the Drosophila peripheral nervous system. They further demonstrate that Lola controls the number, growth and distribution of dendrite branches in da neurons. Loss of Lola also leads to increased expression of Spir, which in turn causes increased formation of abnormal and inappropriately positioned actin-rich branches. In line with this, the authors report that Spir promotes F-actin nucleation and regulates dendrite positioning. Together, these findings suggest that Lola acts to limit the expression of Spir within da neurons, thus ensuring balanced control of the actin cytoskeleton and regulated dendrite morphogenesis.
miR-335 shapes an endoderm transcription factor gradient
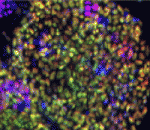 Morphogen and transcription factor gradients are known to pattern tissues during development, but how these gradients are established is unclear. Using mouse embryos, embryonic stem cells (ESCs) and mathematical modelling, Heiko Lickert and colleagues show that the microRNA miR-335 fine-tunes a transcription factor gradient in the endoderm (p. 514). The researchers identify miR-335 as a microRNA that is differentially regulated during mesendoderm differentiation. They further show that miR-335 is expressed and functions transiently in endoderm progenitors and later during mesoderm formation. Importantly, miR-335 targets mRNAs encoding the endoderm-determining transcription factors Foxa2 and Sox17; miR-335 overexpression blocks endoderm differentiation in ESCs and, conversely, inhibition of miR-335 activity leads to Foxa2 and Sox17 accumulation and increased endoderm formation. Finally, mathematical modelling incorporating both microRNA and protein turnover rates predicts that miR-335 can shape a gradient of Foxa2 and Sox17 in the endoderm, and this prediction is confirmed experimentally. Overall, these findings highlight that a microRNA can shape a transcription factor gradient in time and space.
Morphogen and transcription factor gradients are known to pattern tissues during development, but how these gradients are established is unclear. Using mouse embryos, embryonic stem cells (ESCs) and mathematical modelling, Heiko Lickert and colleagues show that the microRNA miR-335 fine-tunes a transcription factor gradient in the endoderm (p. 514). The researchers identify miR-335 as a microRNA that is differentially regulated during mesendoderm differentiation. They further show that miR-335 is expressed and functions transiently in endoderm progenitors and later during mesoderm formation. Importantly, miR-335 targets mRNAs encoding the endoderm-determining transcription factors Foxa2 and Sox17; miR-335 overexpression blocks endoderm differentiation in ESCs and, conversely, inhibition of miR-335 activity leads to Foxa2 and Sox17 accumulation and increased endoderm formation. Finally, mathematical modelling incorporating both microRNA and protein turnover rates predicts that miR-335 can shape a gradient of Foxa2 and Sox17 in the endoderm, and this prediction is confirmed experimentally. Overall, these findings highlight that a microRNA can shape a transcription factor gradient in time and space.
Eyeing up nutrient control of stem and progenitor cells
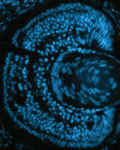 It is known that nutrient availability affects cell proliferation, but how nutrients affect the proliferation-differentiation programme of cells is unclear. On p. 697, Nicola Love and colleagues address this issue, using the ciliary marginal zone (CMZ) of the Xenopus retina as a model. They find that nutrient deprivation (ND) reduces the proliferation, and hence the number, of committed retinal progenitors in the CMZ. By contrast, retinal stem cells at the CMZ peripheral edge are relatively insensitive to ND. Furthermore, ND prevents cells from acquiring a committed progenitor fate, suggesting the presence of a nutrient-sensitive restriction point in the retinal progenitor proliferation-differentiation programme. Finally, the authors show that this restriction point involves mTOR signalling; blocking mTOR mimics many of the effects of ND, whereas activation of mTOR stimulates differentiation. Together, these findings suggest that an mTOR-dependent restriction point in the proliferation-differentiation programme of retinal progenitors exists to couple nutrient availability to tissue growth and development, thus allowing regrowth in ND tissue when conditions of plenty return.
It is known that nutrient availability affects cell proliferation, but how nutrients affect the proliferation-differentiation programme of cells is unclear. On p. 697, Nicola Love and colleagues address this issue, using the ciliary marginal zone (CMZ) of the Xenopus retina as a model. They find that nutrient deprivation (ND) reduces the proliferation, and hence the number, of committed retinal progenitors in the CMZ. By contrast, retinal stem cells at the CMZ peripheral edge are relatively insensitive to ND. Furthermore, ND prevents cells from acquiring a committed progenitor fate, suggesting the presence of a nutrient-sensitive restriction point in the retinal progenitor proliferation-differentiation programme. Finally, the authors show that this restriction point involves mTOR signalling; blocking mTOR mimics many of the effects of ND, whereas activation of mTOR stimulates differentiation. Together, these findings suggest that an mTOR-dependent restriction point in the proliferation-differentiation programme of retinal progenitors exists to couple nutrient availability to tissue growth and development, thus allowing regrowth in ND tissue when conditions of plenty return.
Jaw-dropping differences in the neural crest
 Variation in jaw size has been crucial to the evolution and adaptation of vertebrates. On p. 674, Jennifer Fish, Richard Schneider and colleagues explore the mechanisms by which duck and quail achieve distinct jaw sizes, testing the hypothesis that differences in neural crest (NC) biology contribute to species-specific differences in jaw size. The researchers show that the total sizes of the pre-migratory NC progenitor populations in duck and quail are similar. However, the midbrain region, which generates jaw NC precursors, is wider and shorter in duck owing to an anterior shift in brain regionalisation. Furthermore, they report, more pre-migratory NC precursors are allocated to the midbrain in duck, which gives rise to an increased number of post-migratory NC cells within the duck mandibular arch. Finally, differences in proliferation lead to an increase in the size of the duck mandibular arch relative to that of the quail. Thus, the authors propose, the larger jaw size of duck is the result of at least three distinct developmental events.
Variation in jaw size has been crucial to the evolution and adaptation of vertebrates. On p. 674, Jennifer Fish, Richard Schneider and colleagues explore the mechanisms by which duck and quail achieve distinct jaw sizes, testing the hypothesis that differences in neural crest (NC) biology contribute to species-specific differences in jaw size. The researchers show that the total sizes of the pre-migratory NC progenitor populations in duck and quail are similar. However, the midbrain region, which generates jaw NC precursors, is wider and shorter in duck owing to an anterior shift in brain regionalisation. Furthermore, they report, more pre-migratory NC precursors are allocated to the midbrain in duck, which gives rise to an increased number of post-migratory NC cells within the duck mandibular arch. Finally, differences in proliferation lead to an increase in the size of the duck mandibular arch relative to that of the quail. Thus, the authors propose, the larger jaw size of duck is the result of at least three distinct developmental events.
PLUS…
How to make spinal motor neurons
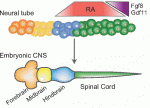 All muscle movements, including breathing, walking, and fine motor skills rely on the function of spinal motor neurons. Here, Kevin Eggan and colleagues discuss how the logic of spinal motor neuron development has been applied to generate motor neurons either from pluripotent stem cells by directed differentiation and transcriptional programming, or from somatic cells by direct lineage conversion. See the Primer on p. 491
All muscle movements, including breathing, walking, and fine motor skills rely on the function of spinal motor neurons. Here, Kevin Eggan and colleagues discuss how the logic of spinal motor neuron development has been applied to generate motor neurons either from pluripotent stem cells by directed differentiation and transcriptional programming, or from somatic cells by direct lineage conversion. See the Primer on p. 491
Lung development: orchestrating the generation and regeneration of a complex organ
 The respiratory system, which consists of the lungs, trachea and associated vasculature, is essential for terrestrial life. In recent years, extensive progress has been made in defining the temporal progression of lung development. This has led to exciting discoveries including the derivation of lung epithelium from stem cells and the discovery of new targets for therapeutics. Michael Herriges and Ed Morrisey highlight review these recent advances in our understanding of lung development and regeneration. See the Review on p. 502
The respiratory system, which consists of the lungs, trachea and associated vasculature, is essential for terrestrial life. In recent years, extensive progress has been made in defining the temporal progression of lung development. This has led to exciting discoveries including the derivation of lung epithelium from stem cells and the discovery of new targets for therapeutics. Michael Herriges and Ed Morrisey highlight review these recent advances in our understanding of lung development and regeneration. See the Review on p. 502


 (No Ratings Yet)
(No Ratings Yet)