In Development this week (Vol. 141, Issue 8)
Posted by Seema Grewal, on 8 April 2014
Here are the highlights from the current issue of Development:
Spine-tingling new role for Sall4
 Wnt, Fgf and retinoic acid signalling play a key role in patterning the posterior neural plate to form the midbrain, hindbrain and spinal cord. Despite intense study of Wnt signalling and neural patterning, only a few target transcription factors that mediate spinal cord development have been identified and the mechanism remains unclear. In this issue (p. 1683), Richard Harland and colleagues reveal a role for Spalt-like 4 (Sall4) in promoting the differentiation of neural progenitor cells in Xenopus via the repression ofpou5f3 (oct4). Morpholino-induced knockdown of Sall4 results in defects in neural tube closure and neural differentiation in the embryo, while morpholino injection at the 4-cell stage reduces expression of spinal cord markers hoxb9, hoxc10 and hoxd10 without affecting pan-neuronal identity. The authors find that when Sall4 activity is disrupted, expression of pou5f3increases, while overexpression of pou5f3 disrupts the expression of key spinal cord identity genes. These data uncover a novel role for Sall4 in neural patterning, with a specific role in spinal cord differentiation.
Wnt, Fgf and retinoic acid signalling play a key role in patterning the posterior neural plate to form the midbrain, hindbrain and spinal cord. Despite intense study of Wnt signalling and neural patterning, only a few target transcription factors that mediate spinal cord development have been identified and the mechanism remains unclear. In this issue (p. 1683), Richard Harland and colleagues reveal a role for Spalt-like 4 (Sall4) in promoting the differentiation of neural progenitor cells in Xenopus via the repression ofpou5f3 (oct4). Morpholino-induced knockdown of Sall4 results in defects in neural tube closure and neural differentiation in the embryo, while morpholino injection at the 4-cell stage reduces expression of spinal cord markers hoxb9, hoxc10 and hoxd10 without affecting pan-neuronal identity. The authors find that when Sall4 activity is disrupted, expression of pou5f3increases, while overexpression of pou5f3 disrupts the expression of key spinal cord identity genes. These data uncover a novel role for Sall4 in neural patterning, with a specific role in spinal cord differentiation.Ageing thymus out-FOXed
 The thymus is central to the adaptive immune system, but it is one of the first organs to undergo an age-related decline in function. Reduced expression of the thymic epithelial cell (TEC)-specific transcription factor FOXN1 has been associated with thymus degeneration, but whether restoration of FOXN1 expression can regenerate an aged thymus is unknown. Now, on p. 1627, Clare Blackburn and colleagues show that provision of FOXN1 in the thymus can reverse fully established age-related thymic degeneration. The authors use an elegant transgenic mouse model to induce the expression of FOXN1 exclusively in the TECs of aged mice, and show that the resulting rejuvenated thymus displays tissue architecture and gene expression similar to that of a much younger mouse. Importantly, the regenerated thymus can generate and export new T cells: a function that is crucial for its role in the adaptive immune system. This is the first report of the regeneration of a whole, aged organ by a single factor and has exciting implications for regenerative medicine.
The thymus is central to the adaptive immune system, but it is one of the first organs to undergo an age-related decline in function. Reduced expression of the thymic epithelial cell (TEC)-specific transcription factor FOXN1 has been associated with thymus degeneration, but whether restoration of FOXN1 expression can regenerate an aged thymus is unknown. Now, on p. 1627, Clare Blackburn and colleagues show that provision of FOXN1 in the thymus can reverse fully established age-related thymic degeneration. The authors use an elegant transgenic mouse model to induce the expression of FOXN1 exclusively in the TECs of aged mice, and show that the resulting rejuvenated thymus displays tissue architecture and gene expression similar to that of a much younger mouse. Importantly, the regenerated thymus can generate and export new T cells: a function that is crucial for its role in the adaptive immune system. This is the first report of the regeneration of a whole, aged organ by a single factor and has exciting implications for regenerative medicine.Muscling in on stem cell hierarchy
 Muscle stem cells, called satellite cells, are responsible for muscle growth and repair throughout life. Different subsets of satellite cells have varying degrees of self-renewal and differentiation potential, but how and when these different subsets arise has not been addressed in vivo. Now, on p. 1649, Andrew Brack and colleagues analyse the precise timing of phenotypic and functional divergence of different satellite cell subpopulations in mouse muscle. The authors use a genetic approach to label satellite cells with an inducible reporter, which becomes diluted with every round of cell division. In this way, the authors identify label-retaining cells (LRCs) that possess greater self-renewal potential than non-LRCs, which are prone to differentiation. The LRCs emerge shortly after birth, become functionally distinct at later stages of postnatal muscle maturation, and are re-established after injury. By comparing slow and fast dividing cells, the authors identify the cell cycle inhibitor p27kip1 as a novel regulator of LRCs, required to maintain their self-renewal potential.
Muscle stem cells, called satellite cells, are responsible for muscle growth and repair throughout life. Different subsets of satellite cells have varying degrees of self-renewal and differentiation potential, but how and when these different subsets arise has not been addressed in vivo. Now, on p. 1649, Andrew Brack and colleagues analyse the precise timing of phenotypic and functional divergence of different satellite cell subpopulations in mouse muscle. The authors use a genetic approach to label satellite cells with an inducible reporter, which becomes diluted with every round of cell division. In this way, the authors identify label-retaining cells (LRCs) that possess greater self-renewal potential than non-LRCs, which are prone to differentiation. The LRCs emerge shortly after birth, become functionally distinct at later stages of postnatal muscle maturation, and are re-established after injury. By comparing slow and fast dividing cells, the authors identify the cell cycle inhibitor p27kip1 as a novel regulator of LRCs, required to maintain their self-renewal potential.Nucleolus precursor body makeover
 Unlike somatic cells, the nucleus of the oocyte and very early embryo contains a morphologically distinct nucleolus called the nucleolus precursor body (NPB). Although this enigmatic structure has been shown to be essential for normal mammalian development, its precise function remains unclear. In this issue, Helena Fulka and Alena Langerova now demonstrate (p. 1694) a crucial role for the NPB in regulating major and minor satellite DNA sequences and chromosome dynamics in the mouse. Absence of the NPB during the first embryonic cell cycle causes a significant reduction in satellite DNA sequences, and the authors also observe extensive chromosome bridging of these sequences during the first embryonic mitosis. The authors further demonstrate that the NPB is unlikely to be involved in ribosomal gene activation and processing as previously believed, since this process can still occur in NPB-depleted early embryos. This study uncovers an interesting and novel role for the NPB in early embryogenesis.
Unlike somatic cells, the nucleus of the oocyte and very early embryo contains a morphologically distinct nucleolus called the nucleolus precursor body (NPB). Although this enigmatic structure has been shown to be essential for normal mammalian development, its precise function remains unclear. In this issue, Helena Fulka and Alena Langerova now demonstrate (p. 1694) a crucial role for the NPB in regulating major and minor satellite DNA sequences and chromosome dynamics in the mouse. Absence of the NPB during the first embryonic cell cycle causes a significant reduction in satellite DNA sequences, and the authors also observe extensive chromosome bridging of these sequences during the first embryonic mitosis. The authors further demonstrate that the NPB is unlikely to be involved in ribosomal gene activation and processing as previously believed, since this process can still occur in NPB-depleted early embryos. This study uncovers an interesting and novel role for the NPB in early embryogenesis.Moss stem cells do it differently
 The WUSCHEL (WUS) family of transcription factors is well known for its role in stem cell maintenance in seed plants. There are two paralogues of the WUS-RELATED HOMEOBOX 13 (WOX13) gene in the moss Physcomitrella patens, but their function is unknown. Now, on p. 1660, Mitsuyasu Hasebe, Thomas Laux and colleagues investigate the role of theWUX13L paralogues in moss and find that the two genes act redundantly to promote stem cell formation, but via a mechanism that differs from that of seed plants. Using a double knockout of the WOX13Lparalogues, the authors show that WOX13L activity is required to initiate the cell growth that is necessary for stem cell formation from detached leaves. Further transcriptome analysis of the double mutant compared with wild-type moss reveals that the WOX13L genes are required for the upregulation of cell wall-loosening genes, revealing a novel function of the WOX gene family.
The WUSCHEL (WUS) family of transcription factors is well known for its role in stem cell maintenance in seed plants. There are two paralogues of the WUS-RELATED HOMEOBOX 13 (WOX13) gene in the moss Physcomitrella patens, but their function is unknown. Now, on p. 1660, Mitsuyasu Hasebe, Thomas Laux and colleagues investigate the role of theWUX13L paralogues in moss and find that the two genes act redundantly to promote stem cell formation, but via a mechanism that differs from that of seed plants. Using a double knockout of the WOX13Lparalogues, the authors show that WOX13L activity is required to initiate the cell growth that is necessary for stem cell formation from detached leaves. Further transcriptome analysis of the double mutant compared with wild-type moss reveals that the WOX13L genes are required for the upregulation of cell wall-loosening genes, revealing a novel function of the WOX gene family.Change of heart for RA signalling
 Retinoic acid (RA) is essential for many developmental processes, but signalling levels must be tightly regulated since too much RA signalling can cause developmental defects. Cyp26 enzymes help to control this balance, metabolising RA and ensuring the correct specification of multiple different organs. Loss of Cyp26 activity can affect heart formation, and now (see p. 1638) Ariel Rydeen and Joshua Waxman reveal a mechanism that may underpin this. The authors show that Cyp26 activity in the zebrafish anterior lateral plate mesoderm (ALPM) is required for the correct specification of cardiac versus vascular lineages. Specifically, loss of Cyp26 activity in zebrafish embryos results in an accumulation of RA and a subsequent increase in the specification of atrial cells at the expense of endothelial progenitors. The authors propose that the Cyp26 enzymes can have non-cell-autonomous consequences through regulating the amount of RA in the local environment to promote vascular specification by defining the boundary between atrial and endothelial progenitor fields in the ALPM.
Retinoic acid (RA) is essential for many developmental processes, but signalling levels must be tightly regulated since too much RA signalling can cause developmental defects. Cyp26 enzymes help to control this balance, metabolising RA and ensuring the correct specification of multiple different organs. Loss of Cyp26 activity can affect heart formation, and now (see p. 1638) Ariel Rydeen and Joshua Waxman reveal a mechanism that may underpin this. The authors show that Cyp26 activity in the zebrafish anterior lateral plate mesoderm (ALPM) is required for the correct specification of cardiac versus vascular lineages. Specifically, loss of Cyp26 activity in zebrafish embryos results in an accumulation of RA and a subsequent increase in the specification of atrial cells at the expense of endothelial progenitors. The authors propose that the Cyp26 enzymes can have non-cell-autonomous consequences through regulating the amount of RA in the local environment to promote vascular specification by defining the boundary between atrial and endothelial progenitor fields in the ALPM.
PLUS…
The evolution and conservation of left-right patterning mechanisms
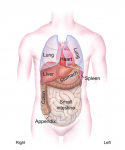 Morphological asymmetry is a common feature of animal body plans, from shell coiling in snails to organ placement in humans. Many vertebrates use cilia for breaking symmetry during development: rotating cilia produce a leftward flow of extracellular fluids that induces asymmetric expression of the signaling protein Nodal. By contrast, Nodal asymmetry can be induced flow-independently in invertebrates. Here, Martin Blum et al ask when and why flow evolved, and propose that flow was present at the base of the deuterostomes and that it is required to maintain organ asymmetry in otherwise perfectly bilaterally symmetrical vertebrates. See the Hypothesis on p. 1603
Morphological asymmetry is a common feature of animal body plans, from shell coiling in snails to organ placement in humans. Many vertebrates use cilia for breaking symmetry during development: rotating cilia produce a leftward flow of extracellular fluids that induces asymmetric expression of the signaling protein Nodal. By contrast, Nodal asymmetry can be induced flow-independently in invertebrates. Here, Martin Blum et al ask when and why flow evolved, and propose that flow was present at the base of the deuterostomes and that it is required to maintain organ asymmetry in otherwise perfectly bilaterally symmetrical vertebrates. See the Hypothesis on p. 1603
The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease
 Over the past 20 years, diverse roles for the Hippo pathway have emerged, the majority of which in vertebrates are determined by the transcriptional regulators TAZ and YAP. Accurate control of the levels and localization of these factors is thus essential for early developmental events, as well as for tissue homeostasis, repair and regeneration. Here, Bob Varelas provides an overview of the processes and pathways modulated by TAZ and YAP and outlines how TAZ and YAP contribute to organ homeostasis and regeneration. See the Review on p. 1614
Over the past 20 years, diverse roles for the Hippo pathway have emerged, the majority of which in vertebrates are determined by the transcriptional regulators TAZ and YAP. Accurate control of the levels and localization of these factors is thus essential for early developmental events, as well as for tissue homeostasis, repair and regeneration. Here, Bob Varelas provides an overview of the processes and pathways modulated by TAZ and YAP and outlines how TAZ and YAP contribute to organ homeostasis and regeneration. See the Review on p. 1614


 (No Ratings Yet)
(No Ratings Yet)