In Development this week (Vol. 142, Issue 20)
Posted by Seema Grewal, on 20 October 2015
Here are the highlights from the current issue of Development:
Atoh1: earmarked for differentiation
 Atoh1 is a key regulator of the differentiation of hair cells, the sensory cells that support audition: it is upregulated during their differentiation and downregulated at postnatal stages. But what are the mechanisms underlying Atoh1 transcriptional regulation during inner ear development? To address this question, Neil Segil and co-workers (p. 3529) analysed the epigenetic status of Atoh1 in mouse hair cell progenitors. They report that histone H3 at the Atoh1 locus is bivalently marked by the repressive tri-methylation of lysine 27 (H3K27me3) and the permissive tri-methylation of lysine K4 (H3K4me3). In nascent hair cells, Atoh1 upregulation is accompanied by a reduction in H3K27me3 and requires the appearance of the permissive acetylation of histone H3 lysine 9. At postnatal stages, Atoh1downregulation is achieved by an increase in H3K9me3, which is a mark indicative of transcriptional silencing, and a reduction in histone H3 acetylation. In early postnatal supporting cells (a cell population that separates and surrounds hair cells and can regenerate them during the first postnatal week in mice), the bivalent marks are maintained, potentially explaining their latent regenerative capacity. This study suggests a mechanism for the epigenetic control of Atoh1 levels during inner ear development and reveals a potential target for future regenerative efforts to replace mammalian hair cells.
Atoh1 is a key regulator of the differentiation of hair cells, the sensory cells that support audition: it is upregulated during their differentiation and downregulated at postnatal stages. But what are the mechanisms underlying Atoh1 transcriptional regulation during inner ear development? To address this question, Neil Segil and co-workers (p. 3529) analysed the epigenetic status of Atoh1 in mouse hair cell progenitors. They report that histone H3 at the Atoh1 locus is bivalently marked by the repressive tri-methylation of lysine 27 (H3K27me3) and the permissive tri-methylation of lysine K4 (H3K4me3). In nascent hair cells, Atoh1 upregulation is accompanied by a reduction in H3K27me3 and requires the appearance of the permissive acetylation of histone H3 lysine 9. At postnatal stages, Atoh1downregulation is achieved by an increase in H3K9me3, which is a mark indicative of transcriptional silencing, and a reduction in histone H3 acetylation. In early postnatal supporting cells (a cell population that separates and surrounds hair cells and can regenerate them during the first postnatal week in mice), the bivalent marks are maintained, potentially explaining their latent regenerative capacity. This study suggests a mechanism for the epigenetic control of Atoh1 levels during inner ear development and reveals a potential target for future regenerative efforts to replace mammalian hair cells.
Revisiting blastomere equality

The fertilised mammalian egg gives rise to seemingly equivalent blastomeres until the fourth cleavage division, when the first indication of lineage specification appears. At this point, certain blastomeres divide symmetrically and others asymmetrically. When do these apparently identical cells diverge and how do these differences arise? To answer this question, Enkui Duan and colleagues performed single-cell transcriptional analysis of human and mouse blastomeres (p. 3468). By studying the mammalian zygote, in which transcription – a known source of heterogeneity during mitosis – is mostly silent, the authors showed that small biases in gene expression arise after the first cleavage division from the unequal distribution of cellular substances between daughter cells, called ‘partitioning errors’. These are especially pronounced for transcripts present in small quantities, which are more likely to be asymmetrically distributed. As cleavage divisions progress, the activation of embryonic transcription minimizes or amplifies the initial biases through positive or negative feedback regulation. Furthermore, the authors show that lineage specification is driven by the relative ratio of pairs of competing lineage specifiers, such as Cdx2 and Carm1, the levels of which are determined by both cleavage history and de novo transcription. This study shows that symmetry breaking leading to lineage specification is a continuous process that emerges as early as the two-cell stage, before morphological differences between blastomeres are detectable.
Revising the origin of thyroid C cells

Thyroid C cells (or parafollicular cells) are neuroendocrine cells found in the thyroid gland that secrete calcitonin. To date, it has been thought that these cells arise from the neural crest but here, on p. 3519, Mikael Nilsson and co-workers overturn this view. Using lineage tracing, they show that Wnt1-positive neural crest cells do not give rise to C cells in the mouse embryonic thyroid gland. Instead, they reveal, thyroid C cells are derived from Sox17-positive anterior endoderm. The researchers further show that the transcription factors Foxa1 and Foxa2, which are known to play a role in the development of other endoderm-derived populations, are co-expressed in C cell precursors, where they play non-redundant roles. Finally, the authors also show that Foxa1 and Foxa2 are expressed and appear to play distinct roles in human medullary thyroid carcinoma (MTC) cells. Together, these findings disprove the current concept of a neural crest origin of thyroid C cells and argue that MTC tumours should be reclassified as neuroendocrine tumours with an endodermal origin, a change that, from a clinical perspective, may open up new avenues in the search for MTC treatments.
Lin-28: balancing stem cell divisions

The Drosophila intestine is known to undergo adaptive growth in response to feeding, and this growth is fuelled by the symmetric self-renewing divisions of intestinal stem cells (ISCs). But what controls ISC division patterns? Here, on p. 3478, Nicholas Sokol and colleagues reveal that the RNA-binding protein Lin-28 promotes symmetric ISC divisions and hence tissue growth in the Drosophila intestine. They first show that Lin-28 is highly enriched in adult Drosophila ISCs. They further report that, although lin-28 null mutants are viable, adult mutant animals exhibit reduced numbers of ISCs. Following on from this, the researchers demonstrate that Lin-28 is required in ISCs to promote food-triggered self-renewing divisions and expansion of the ISC pool. Finally, they report that Lin-28 acts independently of its well-known target let-7 and instead interacts with mRNA encoding the insulin-like receptor (InR), suggesting that Lin-28 modulates InR levels, and thus insulin signalling, to control cell division patterns. In summary, the authors propose that Lin-28 acts as a stem cell intrinsic factor that boosts insulin signalling in ISCs and promotes their symmetric division in response to nutrients.
PLUS:
An interview with Mike Levine
 Mike Levine, director of the Lewis-Sigler Institute for Integrative Genomics at Princeton University, is a developmental biologist who has dedicated his career to understanding how gene expression is regulated during development. We had a lively chat with Mike at this year’s Society for Developmental Biology meeting, where he was awarded the Edwin Grant Conklin Medal. See the Spotlight article on p. 3453
Mike Levine, director of the Lewis-Sigler Institute for Integrative Genomics at Princeton University, is a developmental biologist who has dedicated his career to understanding how gene expression is regulated during development. We had a lively chat with Mike at this year’s Society for Developmental Biology meeting, where he was awarded the Edwin Grant Conklin Medal. See the Spotlight article on p. 3453
Heparan sulfate proteoglycans: a sugar code for vertebrate development?
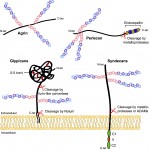 Heparan sulfate proteoglycans (HSPGs) have long been implicated in a wide range of cell-cell signaling and cell-matrix interactions, both in vitro and in vivo in invertebrate models. Here, provide a comprehensive overview of the various roles of HSPGs in these systems and explore the concept of an instructive heparan sulfate sugar code for modulating vertebrate development. See the Review on p. 3456
Heparan sulfate proteoglycans (HSPGs) have long been implicated in a wide range of cell-cell signaling and cell-matrix interactions, both in vitro and in vivo in invertebrate models. Here, provide a comprehensive overview of the various roles of HSPGs in these systems and explore the concept of an instructive heparan sulfate sugar code for modulating vertebrate development. See the Review on p. 3456
Featured movie
This issue’s featured movie shows mouse development from E11.5 to E14.0, as displayed in the 4D atlas of mouse development developed by Wong and colleagues. Read their paper on this useful resource: http://bit.ly/1NeEiAg


 (No Ratings Yet)
(No Ratings Yet)