Inflate your embryo
Posted by Mathieu Le Verge--Serandour, on 17 September 2019
How would you create a hole between two sticky surfaces? Simply crack it!
At a first glance, trying to pull apart the two surfaces seems to be a good idea, but in practice, you might need a lot of energy. However, it seems that the mouse embryo has found a smart and efficient way to do so during its pre-implantation development. After three rounds of cellular divisions, the 8-cell stage embryo starts to compact: cell-cell contacts are expanding, making the embryo more spherical instead of a collection of bubble-like cells [1]. After another round of cleavage, it also internalizes the cells that are more contractile [2]. They will become the Inner Mass Cell (ICM), the future fetus proper, while less contractile cells, the Trophectoderm cells (TE) form a squamous epithelium, that surrounds the ICM and will become part of the placenta. From this step, the embryo is almost spherical, with two layers of cells.
Then, at the 32-cell stage, the embryo shows a new feature: a lumen, a fluid-filled cavity, that breaks the previous radial symmetry by forming at the interface of TE and ICM cells. To grow a lumen, three conditions are needed: 1- to have a sealed compartment, here ensured by the tight junctions between TE cells at the embryo surface; 2- to draw water towards the sealed compartment: in our case, the mouse embryo builds an osmotic gradient by pumping ions in the intercellular medium and lets the water flow through pores; 3- and finally, you have to make room for the accumulated fluid. But here is the problem: the blastocoel forms systematically on the basolateral side of the TE cells, where the cells strongly adhere together! In most other examples of lumen formation, the opening happens at the apical side of the epithelium, where adhesion is repressed! Thus, arises the question: how can you create a lumen at the adhesive side of cells?
In our research [4], we combine developmental biology and physics to decipher the mechanisms of the embryogenesis. We have found that the apparition and the positioning of the lumen, the so-called blastocoel, can be explained using simple physical and biological concepts.
The formation of the blastocoel was a long-time debated topic. Studies have mainly focused on the expansion phase, when the blastocoel is already positioned, while its initiation and positioning are still poorly understood.
What Julien did first, with the help of Francesca and Ludmilla, was to look at the steps preceding the apparition of the blastocoel. In the last decades, efforts have been done on culture conditions and advances in microscopy have permitted to reduce light exposition while improving spatiotemporal resolution. Using resolutive imaging in space and time, that involved the use of both transgenics and microscopy techniques, heobserved the embryo literally boiling!Hundreds of bubbles appeared at the intercellular contacts before the final lumen, forming a network of small microlumens throughout the embryo. Some of those microlumens grew in size, while others disappeared (Fig. 1). As biologist, this observation might seem not significant, but for physicists, this coarsening process immediately rang a bell: looking at the movies, it was really analogous to a well-known process in soft-matter physics: Ostwald ripening. Basically, it describes how in a vinaigrette, the droplets of vinegar will coarsen into fewer drops, the bigger droplets growing to the detriment of the smaller.
From this observation, the collaboration between the two teams emerged, with one team of biologists (Julien, Francesca, Ludmilla and Jean-Léon), the other of physicists (Mathieu, Annette and Hervé), with two questions: i- how these water pockets form in spite of cell adhesion? ii- as they form ubiquitously through the embryo, what mechanisms ensure the formation of a single blastocoel and its final positioning?
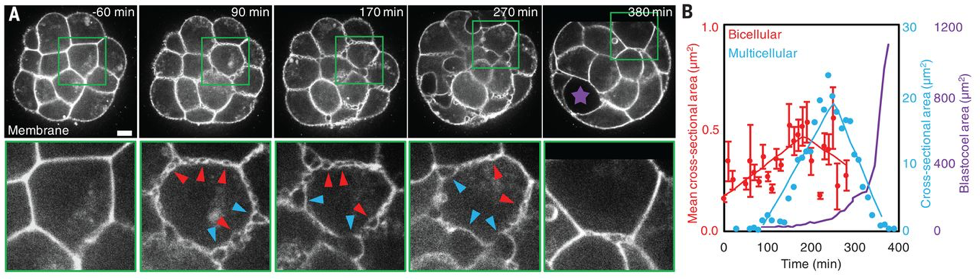
The microlumens form and expand in the extracellular space, at the interface between cells. Julien looked at those cellular contacts, showing that during the formation of microlumens, the spatial distribution of adhesion molecule (E-cadherins) evolves from a homogeneous to a localized heterogeneous distribution. From this observation came the idea of hydraulic fracturing, where water pressure cracks cell-cell contacts exactly like it would crack the rock in oil fracking [3].
After discussions, we came with two main scenarii. a- as cells are active material, they could autonomously regulate their adhesion and create weak points where the fluid could accumulate; b- adhesion is a force that opposes to fluid accumulation, and the expansion of microlumens is capable of pushing adhesion molecules away. To answer this, we had no direct way to measure inside the embryo how cells react against an increase of pressure in the intercellular space. So instead, we chose to inhibit (in three different ways) the formation of the microlumens. in the absence of microlumen, we couldn’t see any reorganization of the E-Cadherin. Our favorite interpretation from this result: this is the hydraulic pressure that breaks locally the adhesion between the cells, and from these breaking points, microlumen can expand. In a nutshell, the embryo seems to generate an increase of hydraulic pressure to break apart all cells contacts instead of specifically regulating its adhesive properties.
A coarsening process is generally made possible by the exchange of matter between different compartments. In the mouse embryo, the microlumens can exchange fluid via the intercellular contacts, which connect them throughout the embryo. A coarsening process akin to Ostwald ripening furthermore involves two other key features: the only stable state is a single droplet, and it requires a surface tension at droplet interfaces, which generate the pressure driving fluid exchange. In the embryo, we invariably observe the formation of a single lumen, and it is furthermore always located at the interface in between the TE and ICM. Thus, we quickly came to the idea that playing on the cell “surface tension” would give us great insights into the mechanical aspects of the blastocoel formation. Indeed, according to previous studies [1], we knew that ICM and TE cells have different levels of contractility, that can physically be translated into surface tensions.
We therefore built a theoretical model of the network of microlumens as a two-dimensional graph of connected hemispherical drops, to test in silico the physical predictions with an algorithm developed by Annette and Mathieu, and we designed experiments to test in situ the biological predictions. Our combined results suggest that, due to osmotic gradient and active pumping, the cells inject fluid that pressurizes the intercellular space, hence creating the hundreds of microlumens by disrupting the cell-cell adhesive contacts. The newly formed microlumens then coarsen into a single final lumen, with a characteristic biphasic dynamic of collective growth then shrinkage, observed both for the model and for the myriad microlumens measured by Julien.
From there, Mathieu predicted with the model the formation of the blastocoel on the side of the embryo, between TE and ICM cells, hence breaking the symmetry of the embryo (Fig. 2., left panel). This prediction was tested using chimeric embryos that Julien made (Fig. 2, center and right panels): an equal mixture of wild-type cells and low-contractility cells, deficient in myosin activity, shows a clear bias for the final position of the lumen toward the low-contractility domain of the chimera, as the theory predicted. The experiments of Julien on low-adhesion mutants, lacking half of E-cadherin activity, also predicted effects of the partial loss of adhesion, that were then tested by Mathieu on the theoretical model, confirming the whole process as being a trade-off between adhesion and contractility.
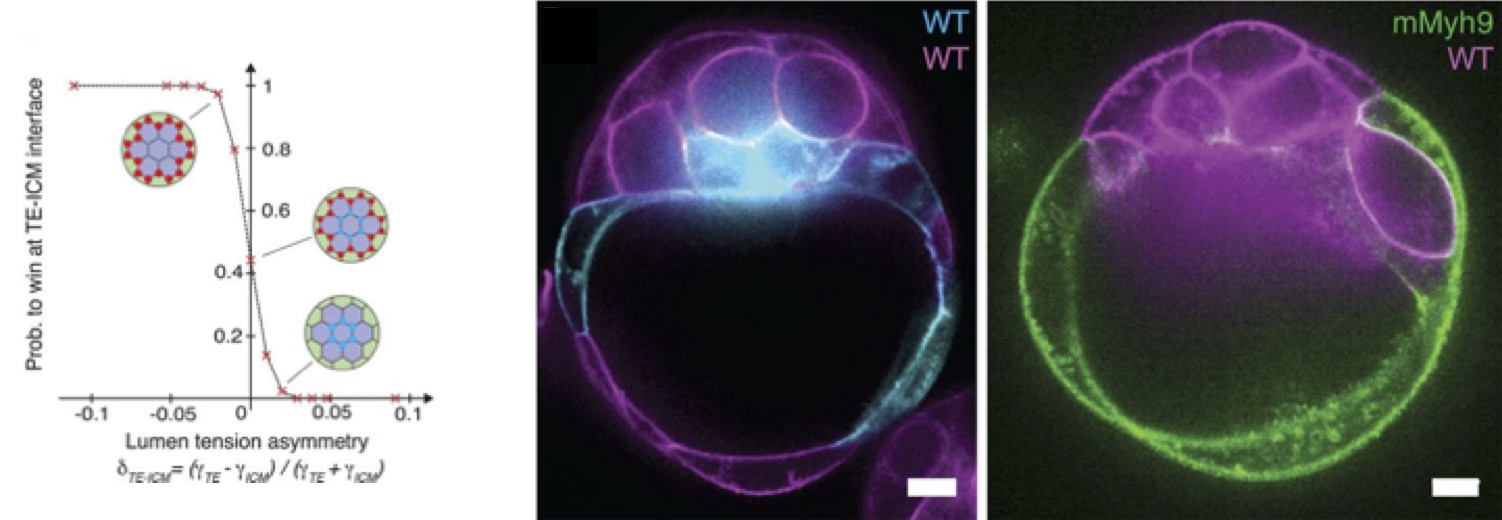
One fascinating aspect of the process is how much it is robust: even though breaking adhesive cell-cell contacts may not be thought to be the best process to form a lumen, the mouse embryo succeeds to form an internal cavity. Moreover, despite the immense molecular complexity of embryo development, the formation of the blastocoel follows rather simple physical mechanisms.
The project was going back and forth between theory and experiment all along. The geographic proximity and the excellent relationship between the two teams were key factors, speeding up the process and the constant exchanges, helping us to remove the barrier between theoretical biophysics and developmental biology.
It was a quick and extremely stimulating project for both our young teams. Part of the pleasure of the project was to gather many people with various expertise, and to see all the pieces matching together to give a comprehensive model at many levels, and of course to work with such enjoyable people.
I, Julien, come from the zebrafish community, and I was used to image embryos that develop fast, as the embryo looks like a fish in 24 hours after fertilization. Then I started to work in Jean-Léon’s team and image relatively slow embryonic development (the mouse embryo takes 3 days to build the blastocyst). Nonetheless, I was convinced that we were missing (and most probably are still missing) key steps of mammalian development and decided to push the system further. Having time resolution of minutes or seconds led to these incredible observations and were key is the direction in which we pushed our research. What I will retain from this work is the exciting collaboration with Annette, Mathieu and Hervé, that opened a new field for me: I must confess, I never heard of coarsening before! I am really happy to see that physicists can be as amazed as developmental biologists by embryogenesis and that these enthusiastic interactions can lead to exciting discoveries.
As far as I (Mathieu) am concerned, this was my first real scientific contribution, ending with a beautiful paper. The fact that such a key step in the mouse embryo development can be simply seen as a fracking and coarsening process still amazes me. Starting the study of the morphogenesis of the mouse embryo was a real challenge with my background of theoretical physicist. Hopefully, Julien and others from his team were always more than happy to speak, and to show me what they were doing, which was an invaluable help. I could not think of better conditions as a start for my PhD, and I am really thrilled to see where it will go.

It opened so many foods for thoughts, promising new and exciting results about the development of the mouse embryo, that we are now trying to push forward. Are there factors that favor the final position within the embryo or is it a stochastic phenomenon? What are the consequences of this increase of pressure on cells at molecular and genetic levels? What triggers the initiation and nucleation of the microlumens?
Julien & Mathieu
References
[2] Maître, Turlier et al., Nature, 2016
[3] Arroyo, Trepat, Science, 2019
[4] Dumortier et al., Science, 2019
Check out the teams !
Team Maître : Mechanics of Mammalian Development (Institut Curie)
Team Turlier : Multiscale Physics of Morphogenesis (CIRB, Collège de France)


 (3 votes)
(3 votes)