My journey to explore the mystery during spermatogenesis
Posted by Mingyao, on 9 November 2023
In a recent Development paper, Wan-Sheng Liu and colleagues find that the cancer/testis antigen PRAMEL1 regulates spermatogonial development by inhibiting retinoic acid signaling, playing a crucial role in the proper establishment of the first and subsequent rounds of spermatogenesis. We caught up with first author Mingyao Yang to find out more about the story behind the paper.
Mingyao, what prompted you to join Wansheng’s lab at the Pennsylvania State University?
During my time at China Agricultural University, I nurtured a profound fascination for reproductive biology. My passion was further ignited through my involvement in a national reproductive lab, where I delved into researching female reproductive biology. As I contemplated pursuing graduate studies in the USA, I came across Dr. Liu’s lab, in the Center for Reproductive biology and Health (CRBH) at Penn State. Although Dr. Liu’s expertise centered on male reproduction, I saw this as a distinctive chance to expand my horizons into uncharted territory. I recognized this opportunity as a platform to enrich my knowledge and skills, providing a stimulating environment for my personal and academic growth. What struck me most was Dr. Liu’s personalized mentorship. He meticulously identified my weaknesses, provided direct guidance, and helped me address each of them individually. This tailored mentorship not only inspired me but also affirmed that I was on the right path to personal growth and a successful research career.
CRBH faculties direct a dynamic and interactive graduate and postgraduate training program and conduct research in diverse areas of reproductive biology and endocrinology. Within this atmosphere, young researchers benefit from interactive learning experiences facilitated by a cohesive team of reproductive experts, engaging in cutting-edge research. In this collaborative space, students, from various labs, use shared equipment to explore diverse scientific questions. The environment fosters extensive discussions, collaborations, and mutual support among our researchers, enriching our educational journey and enhancing our research endeavors. These reasons really attracted me to Dr. Liu’s lab.
How did the project get started?
PRAME (Preferentially Expressed Antigen in Melanoma) was first discovered in melanoma cells in 1997 (Ikeda et al., 1997). Subsequent research revealed that PRAME can multiply across different chromosomes during evolution, forming a multicopy gene family in eutherian animals (Chang et al., 2011). Human, mouse, and bovine genomes contain approximately 60, 90, and 60 copies of PRAME, respectively. Since its discovery, over 500 papers have been published on the Prame family, with most focusing on cancer biology and only a few on reproduction. Our laboratory contributes to unravel the roles of the Prame family in reproduction.
In cancer biology, PRAME serves as a biomarker for various types of cancers (Epping et al., 2005; Kern et al., 2021). Its molecular function involves inhibiting the retinoic acid receptor (RAR) signaling pathway, blocking differentiation, and promoting proliferation (Epping et al., 2005). In germline development, PRAME members (PRAMEL7 and PRAMEL19) counteract retinoic acid (RA)-dependent differentiation, maintaining naïve pluripotency in embryonic stem cells (Casanova et al., 2011; Graf et al., 2017; Napolitano et al., 2020). In spermatogenesis, PRAMEF12 is known to regulate the number of spermatogonia stem cells (SSCs), although its specific molecular functions remain unstudied (Wang et al., 2019).
Previous studies in Dr. Liu’s lab revealed that PRAMEL1 expression is enriched in the testes, particularly in spermatogenic cells ranging from spermatogonia to mature spermatozoa (Liu et al., 2021; Mistry et al., 2013). Based on this information, we hypothesized that PRAMEL1 might be involved in spermatogenesis by inhibiting the RA signaling pathway.
To test this hypothesis, we generated Pramel1 conditional and global knockout mice, forming the basis for this project.
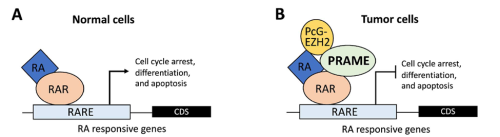
What was known about the role of retinoic acid signaling in spermatogenesis before your work?
Retinoic acid (RA) signaling plays a crucial role in male reproduction and is essential for spermatogenesis (Griswold, 2016). Animals deficient in RA exhibit spermatogonia arrest and infertility. Retinoic acid drives at least four germ cell transitions during spermatogenesis (Endo et al., 2017; Griswold, 2016). In mice, the first transition occurs a few days after birth (around postnatal day 3 (P3)), transforming prospermatogonia into three subtypes of spermatogonia: SSCs (spermatogonial stem cells), progenitors, and A1 spermatogonia (Busada et al., 2014). A1 spermatogonia continue developing to initiate the first round of spermatogenesis, progenitors initiate the second round, while SSCs prepare for subsequent rounds (Law et al., 2019). The first pulse of RA initiates this initial germ cell transition.
Additionally, during each round of spermatogenesis, RA pulses stimulate spermatogonia differentiation, spermatocyte meiosis, spermatid elongation, and the release of spermatozoa from the seminiferous epithelium.
Can you summarize the findings in a paragraph?
In this study, we examined the underlying cellular and molecular mechanisms of PRAMEL1 during spermatogenesis. We reported findings on the involvement of PRAMEL1 in the initiation and maintenance of spermatogenesis by analyzing mouse models with either global or conditional Pramel1 inactivation. We found that:
- Pramel1 plays a crucial role in regulating RA responsiveness of cell-fate committed prospermatogonia, maintaining a balance between undifferentiated and differentiating spermatogonia during the initial round of spermatogenesis.
- Pramel1 has a more pronounced effect on progenitors than on other subtypes of germ cells in young males. It also plays a role in maintaining undifferentiated spermatogonial populations in mature mice.
- PRAMEL1 affects progenitor homing process during the initiation of spermatogenesis in neonatal testis.
- Pramel1 deficiency led to an increased fecundity in juvenile males and decreased fecundity in mature males.
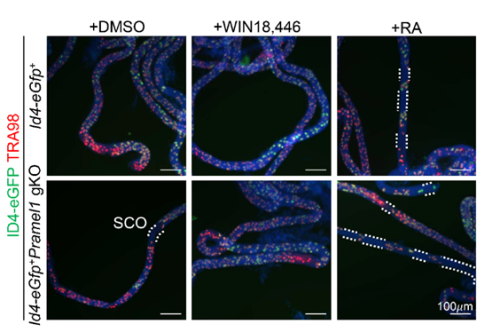
- Pramel1 deficiency resulted in a regional Sertoli cell-only (SCO) phenotype during the first round of spermatogenesis, which was rescued by administration of the RA inhibitor WIN18,446, suggesting that PRAMEL1 functions as an inhibitor of RA signaling in germ cells.
Overall, our findings suggest that PRAMEL1 fine-tunes RA signaling, playing a crucial role in the establishment of the first and subsequent rounds of spermatogenesis.
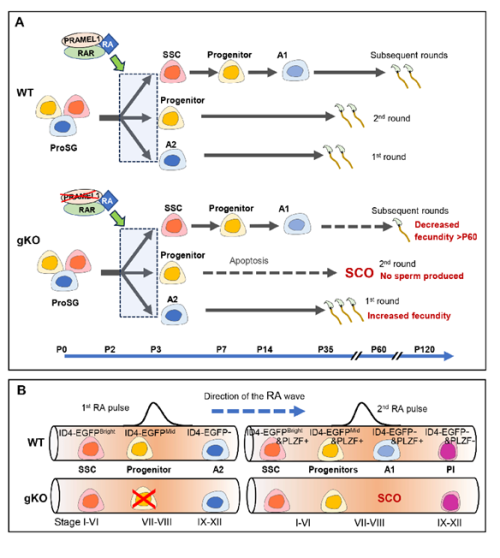
Were you surprised to find that Pramel1 deficiency affected juvenile and mature mice differently?
Certainly, we were surprised by these findings, as we did not anticipate the divergent function of PRAMEL1 in young animals compared to older ones.
Interestingly, a novel concept has emerged indicating that the first round of spermatogenesis constitutes a distinct program separate from the subsequent rounds (Law et al., 2019; Yoshida et al., 2006). During the first round, sperms are produced at a juvenile age, whereas the subsequent rounds of spermatogenesis occur during mature age. The initial A2 spermatogonia, transitioning directly from prospermatogonia in response to the first RA pulse, drives the first round of spermatogenesis. In contrast, the subsequent rounds of spermatogenesis originate from spermatogonial stem cells (SSCs). Our results provide compelling evidence supporting the idea that the mechanisms underlying the first round and subsequent rounds of spermatogenesis are different.
Did you have any particular result or eureka moment that has stuck with you?
During this work, I often felt like walking in a maze. There are too many unsolved mysteries during spermatogenesis. However, fresh results and inventive ideas, whether derived from literature, expert insights, or our own discussions, served as beacons of guidance, illuminating our path, and bringing moments of clarity amidst the intricate complexity.
One of the exciting moments was when we obtained the whole-mount immunofluorescence (IFS) results following RA treatment. These results revealed that the RA inhibitor successfully rescued the regional SCO phenotype in the young gKO testis. This outcome strongly suggested that PRAMEL1 acts as an inhibitor of RA signaling during spermatogenesis. Typically, my advisor, Dr. Wansheng Liu, and I often have different interpretations or perspectives regarding my results. However, this time, he wholeheartedly agreed with me when we examined the original results, and our shared enthusiasm underscored the significance of our findings.
And the flipside: were there any moments of frustration or despair?
Certainly, graduate school, especially for international students like me, came with its fair share of frustrating moments. One such instance involved the extensive immunofluorescence staining required for our research paper. Initially, the staining procedures didn’t yield high-quality results, possibly due to issues with our protocol or the antibodies we were using. I persisted in optimizing our protocol, conducting the staining repeatedly in an attempt to improve the outcomes. Simultaneously, I experimented with numerous antibodies sourced from different companies. Complicating matters, our funding was limited at that time, requiring us to approach these companies and request free samples of antibodies, which we tested one by one. The journey to completing this project was arduous, but it was also incredibly motivating to witness the quality of results improving gradually with each attempt.
What’s next for this story? And personally, Mingyao, what is next for you after this paper?
In this study, we understand the role of PRAMEL1 during spermatogenesis while our previous study has revealed? the function of PRAMEX1 in testis. To gain a better understanding of the role of PRAME family during spermatogenesis, we have successfully generated a Pramel1/Pramex1 double knockout mice. Thus, the next of this story is to figure out the interaction of the two different members of Prame family during spermatogenesis.
For me, I will further explore the mystery during spermatogenesis in the lab of Dr. Oatley (one of our co-authors in this paper) in Washington State University. My career goal is to become an independent investigator researching the mechanisms that underpin spermatogenesis. My hope is that the outcomes of my research program will be translated to solutions for male infertility that impacts humans, domestic animals, and wildlife. Infertility is a significant concern that affects a substantial number of people worldwide, with approximately 20% of couples facing difficulties conceiving a pregnancy. Through my research in male reproductive biology, I aim to contribute to the development of innovative solutions and interventions to address the male side of infertility. This involves investigating the underlying causes of male infertility, treatment options, and improving assisted reproductive technologies. By gaining a deeper understanding of reproductive processes and disorders, I hope to make meaningful contributions to improving fertility outcomes and enhancing the quality of life for individuals and families facing fertility challenges.
Reference:
Busada, J. T., Kaye, E. P., Renegar, R. H., & Geyer, C. B. (2014). Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biology of Reproduction, 90(3), 1–11. https://doi.org/10.1095/biolreprod.113.114645
Casanova, E. A., Shakhova, O., Patel, S. S., Asner, I. N., Pelczar, P., Weber, F. A., Graf, U., Sommer, L., Bürki, K., & Cinelli, P. (2011). Pramel7 mediates LIF/STAT3-dependent self-renewal in embryonic stem cells. Stem Cells, 29(3), 474–485. https://doi.org/10.1002/stem.588
Chang, T., Yang, Y., Yasue, H., Bharti, A. K., Retzel, E. F., & Liu, W. (2011). The Expansion of the PRAME Gene Family in Eutheria. 6(2). https://doi.org/10.1371/journal.pone.0016867
Endo, T., Freinkman, E., De Rooij, D. G., & Page, D. C. (2017). Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America, 114(47), E10132–E10141. https://doi.org/10.1073/pnas.1710837114
Epping, M. T., Wang, L., Edel, M. J., Carlée, L., Hernandez, M., & Bernards, R. (2005). The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell, 122(6), 835–847. https://doi.org/10.1016/j.cell.2005.07.003
Graf, U., Casanova, E. A., Wyck, S., Dalcher, D., Gatti, M., Vollenweider, E., Okoniewski, M. J., Weber, F. A., Patel, S. S., Schmid, M. W., Li, J., Sharif, J., Wanner, G. A., Koseki, H., Wong, J., Pelczar, P., Penengo, L., Santoro, R., & Cinelli, P. (2017). Pramel7 mediates ground-state pluripotency through proteasomal-epigenetic combined pathways. Nature Cell Biology, 19(7), 763–773. https://doi.org/10.1038/ncb3554
Griswold, M. D. (2016). Spermatogenesis: The commitment to Meiosis. Physiological Reviews, 96(1), 1–17. https://doi.org/10.1152/physrev.00013.2015
Ikeda, H., Lethe, B., Baren, N. Van, Smet, C. De, Vitale, M., Moretta, A., Boon, T., Coulie, P. G., Istologia, I., & Biomediche, S. (1997). Characterization of an Antigen That Is Recognized on a Melanoma Showing Partial HLA Loss by CTL Expressing an NK Inhibitory Receptor. Immunity, 6, 199–208.
Kern, C. H., Yang, M., & Liu, W. S. (2021). The PRAME family of cancer testis antigens is essential for germline development and gametogenesis. Biology of Reproduction, 105(2), 290–304. https://doi.org/10.1093/biolre/ioab074
Law, N. C., Oatley, M. J., & Oatley, J. M. (2019). Developmental kinetics and transcriptome dynamics of stem cell specification in the spermatogenic lineage. Nature Communications, 10(1), 1–14. https://doi.org/10.1038/s41467-019-10596-0
Liu, W. S., Lu, C., & Mistry, B. V. (2021). Subcellular localization of the mouse PRAMEL1 and PRAMEX1 reveals multifaceted roles in the nucleus and cytoplasm of germ cells during spermatogenesis. Cell and Bioscience, 11(1), 1–18. https://doi.org/10.1186/s13578-021-00612-6
Mistry, B. V, Zhao, Y., Chang, T., Yasue, H., Chiba, M., & Oatley, J. (2013). Differential Expression of PRAMEL1 , a Cancer / Testis Antigen , during Spermatogenesis in the Mouse. 8(4). https://doi.org/10.1371/journal.pone.0060611
Napolitano, G., Tagliaferri, D., Fusco, S., Cirillo, C., De Martino, I., Addeo, M., Mazzone, P., Russo, N. A., Natale, F., Cardoso, M. C., De Luca, L., Lamorte, D., La Rocca, F., De Felice, M., & Falco, G. (2020). A novel member of Prame family, Gm12794c, counteracts retinoic acid differentiation through the methyltransferase activity of PRC2. Cell Death and Differentiation, 27(1), 345–362. https://doi.org/10.1038/s41418-019-0359-9
Wang, Z., Xu, X., Li, J. L., Palmer, C., Maric, D., & Dean, J. (2019). Sertoli cell-only phenotype and scRNA-seq define PRAMEF12 as a factor essential for spermatogenesis in mice. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-13193-3
Yoshida, S., Sukeno, M., Nakagawa, T., Ohbo, K., Nagamatsu, G., Suda, T., & Nabeshima, Y. (2006). The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. 1505, 1495–1505. https://doi.org/10.1242/dev.02316


 (No Ratings Yet)
(No Ratings Yet)