New signal revealed for birth of blood stem cells in vertebrates
Posted by StJude, on 1 March 2017
Jamie R. Genthe and Wilson K. Clements
When blood goes bad, a replacement is often needed. Each year, thousands of patients in the US receive bone marrow transplants to treat life-threatening diseases like blood cancer.
But in some cases, the transplant itself can become deadly.
The problem is not necessarily the one most people think of: rejection of the transplanted material. Instead, the donor hematopoietic stem cells (HSCs), delivered to repopulate the whole blood system, sometimes produce immune cells that attack anything foreign. In these cases, the transplant rejects the patient. This condition, called graft-versus-host disease (GVDH), is common in transplant patients and often fatal.
Developing a reliable method to make transplantable stem cells that would avoid GVHD is of considerable interest. That’s why we and others have been interested in learning how to direct the differentiation of HSCs in vitro from pluripotent precursor cells.
Work from our group at St. Jude Children’s Research Hospital, published in the February 15 issue of Development, provides new clues on how to help solve this problem.
Intuitively, a way to make new HSCs would be to replicate the same instructions used to make them in the developing embryo. But first we have to identify the signals that make up those instructions. During embryonic development, HSCs are born from the descending aorta, the primitive vessel that carries blood to the lower half of the body. Decades of research have uncovered numerous signaling inputs that direct formation of the descending aorta, and its subsequent conversion to the first HSCs. However, we are still defining the complete set of signals, and how they are regulated and integrated.
We set out to examine this coordination and identify novel factors that might play critical roles in HSC development. We used zebrafish because their blood development is nearly identical to that of humans. We noticed a common theme: signaling pathways required for hematopoietic stem cell formation also frequently regulate vessel patterning. Specifically, well-established signaling pathways, like the Vegf, Notch, and Wnt pathways, play a role in both processes.
In the trunk of the embryo, smaller blood vessels called “intersegmental vessels” sprout from the developing descending aorta at about the same time that HSCs are born. We became intrigued by published results identifying a new signaling pathway necessary for the sprouting of intersegmental vessels from the aorta (Gore et al., 2011). This pathway involves R-spondin-1 (Rspo1), a secreted factor that augments Wnt signaling. Intriguingly, Gore et al. also showed rspo1 was expressed in the dorsal aorta during the time of HSC specification, the point when blood stem cells start to assume their future identity. We wondered if this pathway might also be necessary for HSC specification.
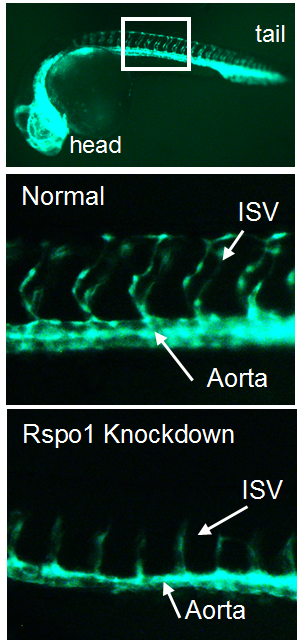
Our first goal was to determine if Rspo1 is required in HSC development. We knocked down Rspo1 in zebrafish embryos using antisense oligonucleotides and asked what would happen to HSCs. Strikingly, markers of HSC development were visibly decreased at all time points we examined. We subsequently confirmed the rspo1 requirement in an established zebrafish hypomorphic mutant.
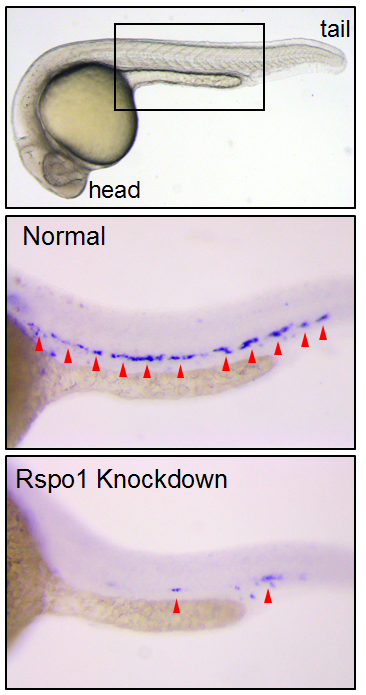
So Rspo1 is required for HSCs to form, but how does it work? To answer this question, we looked at the activity of other pathways already known to play a role in HSC specification to see if we could detect any changes in their activity. Our results identified two of these – the Wnt16 and Vegfa pathways – that showed alterations in expression of their downstream components.
Our findings had some surprises. Most notably, although vegfa expression was lower when Rspo1 was knocked down, a key target of Vegf signaling, notch1b, was unaffected. Our finding suggested that there might be a Notch1-independent role for Vegf in specifying hematopoietic stem cells.
As we were trying to define how this might work, the group of Roger Patient (Monteiro et al., 2016) identified precisely such a pathway. We determined that Rspo1 acted via this Notch-independent pathway, and discovered that a particular Vegfa splice variant was involved.
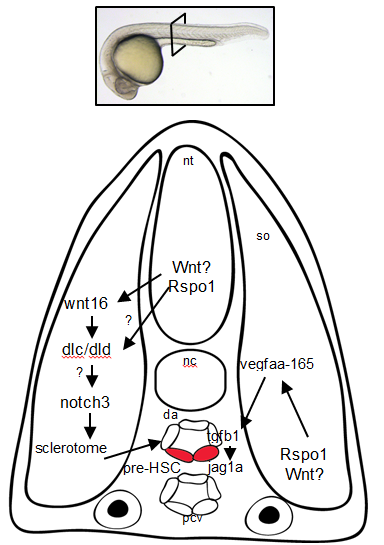
Overall, these findings point to Rspo1 as a new master regulator of blood stem cells that controls two pathways both needed for the birth of these stem cells. In the future, we hope that understanding the full set of signals and their integration will provide the key to unlocking our ability to make fully functional hematopoietic stem cells in the lab. Eventually we will have the tools to create designer treatments for leukemia and other blood disorders while making GVHD a thing of the past.
Comment on
R-spondin-1 is required for specification of hematopoietic stem cells through Wnt16 and Vegfa signaling pathways”. Development 2017, .
References
Gore, A.V., Swift, M.R., Cha, Y.R., Lo, B., McKinney, M.C., Li, W., Castranova, D., Davis, A., Mukouyama, Y.S., Weinstein, B.M., 2011. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138, 4875-4886.
Monteiro, R., Pinheiro, P., Joseph, N., Peterkin, T., Koth, J., Repapi, E., Bonkhofer, F., Kirmizitas, A., Patient, R., 2016. Transforming Growth Factor beta Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev Cell 38, 358-370. [*Lead author wrote about this work on the Node last year*]


 (4 votes)
(4 votes)