Non-model organisms: weird critters and the people who study them
Posted by Alexandra Bisia, on 29 January 2024
By Brent Foster and Alexandra Bisia
When the two of us, Brent and Alex, started our Node correspondent positions early last year, we both expressed an interest in non-model organisms (NMOs). While one of us (Brent) works with several NMOs, the other (Alex) is solidly in the mouse camp, arguably the ur-model organism of our days. Since we both declared a shared interest in NMOs, it was a matter of competing or collaborating (har har)! Combining our forces, we identified and individually interviewed five researchers who work with NMOs in their labs. But even in this group, some NMOs are more conventional than others.
Here, we have condensed each individual interview into one “super-interview” that compares and contrasts the questions, the approaches, and the problems these labs encounter in their research. The result is a regular pot pourri of NMO research. As they all understandably had many interesting things to say about their respective research areas, we’ll be uploading individual interviews as articles for The Node throughout the next couple of months.
It’s critical to establish systems facilitating investigations in novel organisms given the urgency to document and understand the rich biodiversity of our natural world and preserve it as best we can. But on top of that, we found these scientists are motivated by a universal human urge to explore something unusual, confusing, or sometimes just plain cool. They demonstrate the true spirit of research.
Although by no means a comprehensive or even representative list of NMOs, we hope this article will encourage people to become familiar with more obscure research models and questions, and perhaps provide the impetus for the field to explore beyond its established comfort zone.
With that, let’s introduce our researchers and their respective NMO(s) of choice:
.

.
.
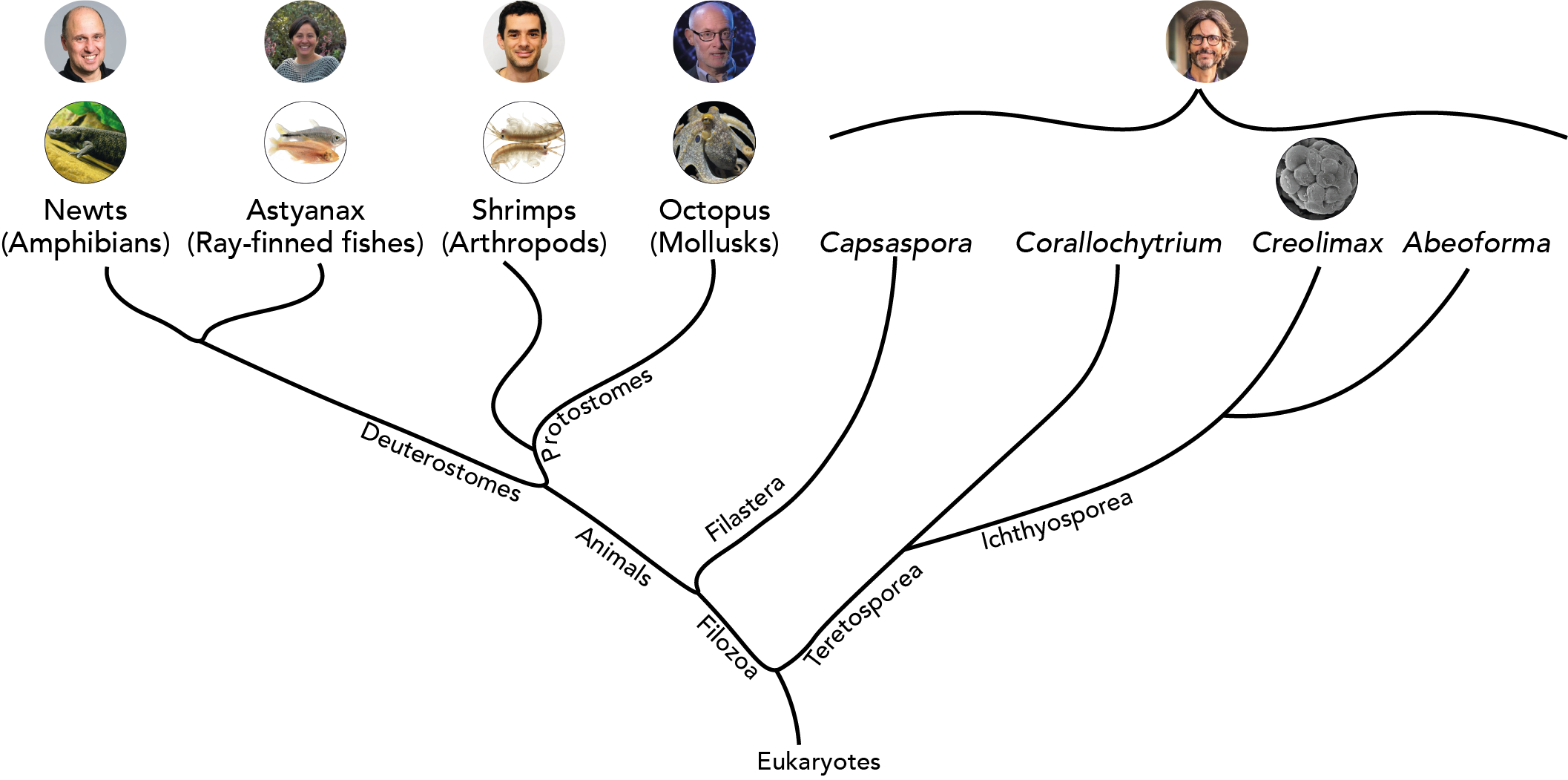
Iñaki Ruiz-Trillo: works with single-celled eukaryotes (Institut de Biologia Evolutiva, Barcelona, Spain)
.
.
.


.
.
Cliff Ragsdale: works with octopus (University of Chicago, USA)
.
.
.


.
.
András Simon: works with newts (Karolinska Institutet, Stockholm, Sweden)
.
.
.


.
.
Michalis Averof: works with shrimps (Institut de Génomique Fonctionelle de Lyon, France)
.
.
.


.
.
Patricia Ornelas-García: works with Mexican cavefish (Universidad Nacional Autónoma de Mexico)
.
.
Could you give us a short summary of your research?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
What fascinates me is the evolutionary transitions. I did my PhD in one of the major evolutionary transitions—the original bilaterian, that transition from radial symmetry to bilaterally symmetrical animals. I was looking to do a postdoc on the origin of eukaryotes. My supervisor proposed to me, saying, ‘I have a very weird organism that seems to be closely related to animals. Would you be interested in the origin of animals?’ I said yes, I’d be interested, and then I started to work.

Cliff Ragsdale (octopus)
I did a post-doc on amphibian limb regeneration. In my own lab I moved into neuroembryology—essentially chick molecular experimental biology focusing primarily on midbrain development. And then about 2 decades ago, I switched to evolutionary neuroscience and was interested in two problems: one, the evolution of the neocortex and the other is on cephalopods, specifically octopus. I was interested in how a large brain could be organized apart from the vertebrate design.

Michalis Averof (shrimp)
In the lab we study regeneration using this small crustacean [Parhyale hawaiensis] as our model. Ever since my PhD, I was interested in comparative developmental biology, in what different organisms can tell us about mechanisms of development and how those mechanisms evolve. This is how we started to work with crustaceans. Then gradually regeneration, which was a side project, became our main focus.

Patricia Ornelas-García (Mexican cavefish)
I work mainly with the systematics and speciation mechanisms in freshwater fish species. Since my PhD, I have worked with Astyanax. In the beginning, I just wanted to [study] as many [Astyanax populations from various] caves as we could, so we could test these hypotheses of how many times the fish has been able to adapt to the caves. Nowadays, we are starting to move to some developmental analyses. So far, we have been able to reproduce five [Astyanax] populations, different from the common ones like Pachón or Tinaja. We are trying to compare the differences during early development. We are also exploring the phenotypic convergence with other cavefish in Mexico, particularly in some processes related with asymmetry.

András Simon (newt)
I started my lab 2002, and since then we have been working on newt regeneration. The reason we are interested in newts is because of their regenerative capacity. They can regenerate large body structures such as entire limbs, large parts of the brain, spinal cord, cardiac muscle. We think that [by studying newts] one can understand mechanisms that allow or disallow regeneration of complex body parts in vertebrates, but also use regeneration as sort of a way to address fundamental cell and molecular or biological questions that these animals are specialists in. In that context, dedifferentiation of cells is one of the main interests in my group.
.
What are some of the particular challenges of using this NMO/NMOs in general?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
There was a lot of missing information. Imagine you want to understand the origin of World War II. You need to have information of how it was before World War II, you know? It’s more or less the same—you need a comparison framework. And there was no information.

Cliff Ragsdale (octopus)
There are still many, many technical challenges. Octopus bimaculoides–the southern California two spot octopus–is kind of like a lab rat in that it has too long of a generation time to be appropriate for any kind of forward genetic studies. We really don’t have a cephalopod yet that’s appropriate for that. And you might say, “Oh, well, why can’t you just use CRISPR?” But the key thing is being able to inject embryos and to have the embryos survive. Transgenesis just hasn’t fallen yet.
There are no invertebrate marine cell cultures. Let that settle in for a moment. You can do primary cultures, and like a science fiction film, these cells in culture or explants in culture will seem to live forever. But it’s very hard. To get proper cell culture of any marine invertebrate would open up a lot of cell biology if the techniques were general ones. There’s hardly anyone working on that, but that’s certainly a need.

Michalis Averof (shrimp)
[The Parhyale research community is] more isolated in terms of technology, in the sense that for every project we have to develop our own tools, there isn’t this big community behind you generating Gal4 drivers or Cre lines that are shared, like you have in other systems. When you start a project, you have to generate those tools by yourself. And that is a major limitation when working with our kind of peripheral models.
The other issue is, we haven’t yet figured out an easy way of sending these animals across the world without having problems with customs.
The benefit, on the other side, is that in almost anything you study you’re going to make new discoveries, because no one has studied that before. So you’re entering a virgin field. You have the opportunity to shape your research field to a larger extent.

Patricia Ornelas-García (Mexican cavefish)
Yeah, I think one of the challenges is that there are a very restricted number of people working with these [species]. And in a way, it’s fascinating, because you will find something new for sure. But [from another point of view], in research groups [studying established organisms] it is easier.
We were trying to characterise the microbiome of the fish. And it was a little challenging, because there was not a lot of information already published on protocols or how to treat the data. Or when we are trying to set up [experiments], for example, for physiology or for another kind of ecological analysis, it’s sometimes difficult.

András Simon (newt)
[Newts are] technically challenging for several reasons. One is the relatively long generation time, which can range from nine months to five years. When the molecular biology and the genetic era entered, these organisms became a bit obsolete. Most researchers said, “Well, if I can do all these things in mice, I won’t do that in salamanders, because it’s just too time consuming.” And funding agencies are not always very patient. They also have quite large genomes. Genome sequencing technologies were not there some years ago to make it possible to get a reasonable assembly of the genome.
.
What are some of the unique opportunities from using NMOs? Or what recent technical developments are aiding research progress?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
Most of the people who were working on the origin of animals were working with early branching animals, like cnidarians, ctenophores, and sponges. This is very important for early animal evolution, but you can never understand the transition because you are not considering the stuff before.

Cliff Ragsdale (octopus)
You know, it’s great to have a genome.

Michalis Averof (shrimp)
[Parhyale] is a very good system if you want to image what happens during regeneration. You can image the whole process at cellular resolution from beginning to end, [and] the reason is that these animals as adults are transparent. They’re small enough that we can just image through their legs with a confocal microscope. We can make transgenics so we can label the cells; and we can immobilise them, which can be a big challenge in other organisms [that regenerate as adults]. In our system, what allows us to image regeneration is the fact that arthropods are encased in the chitinous exoskeleton. We can use simple surgical glue to stick those animals onto a cover slip. And they will stay there, they can’t go away until they molt.

Patricia Ornelas-García (Mexican cavefish)
During my Bachelor’s, I was working with mice, the typical model in the lab, and somehow I think that the number of questions sometimes can be very restricted because there’s already so many studies in these animals, that it’s difficult to come up with something new.
And [working with Astyanax] I have a lot of things in my favour, I am from Mexico, I can work in the field, I can do a lot of in situ experiments. Even nowadays, there are very few Mexicans working with Astyanax. It just happened that there were a lot of things that made me realise that there was a lot of potential in the [Astyanax] system for me.

András Simon (newt)
For us, there were three things that enabled us to interrogate the problem at the molecular level. First, we introduced to the lab a new species, Pleurodeles waltl, which is relatively easy to breed. Secondly, sequencing technologies improved to the degree that now we can compile a good genome assembly. The third is genome editing technology. CRISPR revolutionised many fields, and ours as well. During the past 7-8 years we have been generating genetically modified animals for cell tracking experiments, for loss-of-function experiments, of different genes. Now we are really in the position to address the question of regeneration with molecular tools, and get some mechanistic understanding at the molecular level.
.
What is a particular study or finding in your NMO of choice that you think is really cool?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
The most important thing we have discovered is that the unicellular ancestor of animals was genetically much more complex than previously thought.

Cliff Ragsdale (octopus)
It’s in the genomes where I think the greatest surprises have appeared. Having large genomes kind of raises the possibility that, like the vertebrate lineage, you have whole genome duplications. That’s something we see in other animals as well. And that doesn’t show up at all in cephalopods. Lots of key gene families are duplicated. We’re very tempted to think, that even though bony fish, some clades have way, way too many genome duplications, we’d like to think that somehow the two rounds of genome duplication leading to jawed vertebrates is important for the innovations we see in structures of vertebrates, including us—there’s no indication of that in cephalopods. That raises the interesting possibility that gene regulatory mechanisms, cis regulatory mechanisms in particular, by disruption of this synteny by this blender effect might underlie many of the innovations seen in soft-bodied cephalopods.

Michalis Averof (shrimp)
One of the latest papers to come out is [by] people who study biological rhythms, and have studied how Parhyale regulate their daily activities in relation to tides. Our animal is an intertidal species, and it seems it has an endogenous clock that runs with the tidal cycle rather than with a day-night cycle. Well, they have both, but somehow the two interact in a complex way. People who study circadian rhythm had noticed that there were two peaks of activity, one in the morning and one in the evening. And the intertidal cycle is a little bit longer than 12 hours. So that might reflect the fact that they have a 12-hour cycle rather than a 24-hour cycle. But of course, in nature, the tidal cycle comes slowly out of sync with the day-night cycle. And that is not observed in the lab. [So] people are beginning to study phenomena that were not accessible before in the standard models, like tides and regeneration. There are new aspects of biology that become accessible once you have a new system.

Patricia Ornelas-García (Mexican cavefish)
In our most recent paper, by a Master’s student of mine, we assess [this] question [of how many times Astyanax has been able to adapt to caves independently]. In our results, we have at least three independent colonisation events of the caves, which for some is crazy, it’s not possible. But from our point of view, we are really relying on exhaustive sampling of the caves, and that is what we’re suggesting.

András Simon (newt)
There’s an interesting dichotomy: one would expect that the price newts pay for getting cells proliferating and dedifferentiating is that they are more prone to get cancer, but that’s actually not true. In fact, during regeneration some genes, which are heavily mutated in mammalian cancers, are actually downregulated during regeneration. And instead of everything just growing randomly, they create a structure.
.
How does data analysis and sharing between labs employing NMOs differ compared to studies using established MOs?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
We have quite a lot of collaborations. Our lab is quite diverse. We collaborate a lot. So far most of the community is quite open. It’s usually not a problem.

Michalis Averof (shrimp)
We all use the same population of Parhyale. It’s a population that has been kept in the lab for more than 20 years. There are a few people beginning to isolate new populations from the wild. The funny thing about the population we share is that it was picked up in an aquarium in Chicago, about 25 years ago, and we don’t know which part of the world it came from originally. We all use it, the genome has been sequenced from and all our transcriptomic work is based on that population. For transgenesis and CRISPR, we more or less use the same protocols. We don’t share transgenic lines so often, but that’s mostly because we have different interests, and each of us develops our own lines for the particular questions we’re asking.

Patricia Ornelas-García (Mexican cavefish)
In my opinion, one of the things that the Astyanax model has is that [researchers working on it] are very open. For example, when we were trying to reproduce a fish, we were [asking for] a lot of information [from other groups].

András Simon (newt)
Personally, I’m very open with our data. If someone asks me about even unpublished data, I’m happy to share. In my group, we sometimes host guest researchers from the field who are welcome to participate in our lab meetings, where we discuss raw data. Of course, sometimes people are more cautious. But I would say in general, this is a super friendly field.
.
How large/connected is the community studying your particular NMO of interest?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
There’s quite a lot of research and quite a lot of labs in the region of multicellularity. My feeling is that every year there is more and more people interested. So now, we are kind of explosive. I see more and more labs connecting with us and being interested in our organisms or in our questions.
It’s one of the biggest transitions compared to all the other major evolutionary transitions. The origin of life, the origin of the eukaryotic cell, the origin of humans, the origin of bilaterian animals–there’s only one single origin of all of that. Animals acquired multicellularity from some kind of ancestor, which we are trying to get who they are, or how they were. Plants acquired multicellularity from a different ancestor from different parts of the tree of life. And algae acquired multicellularity from different ancestors with different genomes, different raw materials. And so on and so on. And the people working on those multicellular organisms—we don’t talk to each other. We go to different conferences, different journals. I think we have to talk to each other at some point. It was impossible to do that in the past because there was a lot of research in the origin of animals, but not so much in the origin of plants. But now there is more and more of these models. I think it’s a very good moment.

Cliff Ragsdale (octopus)
There’s a huge explosion with people who are interested in the brain or who are interested in development and other aspects of cephalopod biology. I kind of think people were always fascinated by cephalopods, captivated by the behaviors of octopus and the adaptive coloration system particularly pronounced in octopuses and cuttlefish.

Michalis Averof (shrimp)
It’s a very small community, there are maybe 20 or 30 people working on the animal. You would imagine that small communities are very well connected. We are connected, but not very tightly. I think it mostly has to do with the fact that we are on different continents and we study different questions. We talk to each other and we share tools and genetic resources. For example the genome sequencing and assembly was a collective effort.

Patricia Ornelas-García (Mexican cavefish)
An important thing to highlight in Astyanax is that we have the biennial meeting. Because it’s not a model organism, [everyone is] really [willing] to talk about the system in a very open way, and include new researchers. Particularly for me, when I was finishing my PhD, this was a very dramatic point, because I saw a potential in the system that I can be included.
Nowadays, a lot of people are trying to [investigate] this model [from] the eco-evo-devo perspective. They have realised [that it is important to distinguish between the different Astyanax lineages], because some of the results that they get are related with the lineage, and not particularly with the environment. In these terms, there is a growing number of labs wanting to work in the field, know more about the ecology in situ, learn more about the behaviour, the physiological adaptations.

András Simon (newt)
We are growing to the degree that we have a yearly salamander meeting. Also, there is the larger field of regenerative biology, which is at a decent size now. We just started a new organisation, the International Society for Regenerative Biology, and the inaugural meeting was in Vienna this year. That gives room to different regeneration model overlays, because regeneration at this scale is quite widespread evolutionarily but also randomly distributed. It doesn’t decline with the increase of [organismal] complexity. At these meetings we gather researchers who work with regeneration model organisms, in particular salamanders, including newts, planaria, zebrafish, but also Hydra, cnidarians.
.
Would you say it takes a particular sort of character to want to study NMOs?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
The most important thing is the question. Then the techniques and the approach vary depending on the moment.
Sometimes it has been hard to be alone. And at the same time, there is something beautiful about that, because you seem to be a pioneer. It takes something of the more human angle of being an explorer, you know? So I cannot complain. I mean, I complain because it was hard at some points and it would have been faster [in another organism]. But also it was nice to, for many years, say, ‘Oh, you should look at these organisms.’ And maybe some people didn’t believe you and now they say, ‘Oh yeah, that’s cool.’

Michalis Averof (shrimp)
Definitely, it takes a different kind of researcher. To work with these animals, you have to realise that research is going to move forward much more slowly, because you will have to start many things from scratch.

András Simon (newt)
If one is not super naïve, it must be that they are more dedicated to answering a question than just progressing in their career. But I like the field a lot. Because of the lack of tools, I found the field intellectually very mature. People had time to think, that is my impression, and I liked it. Now, of course, there are [more] tools. So the risk is that we’re going to do too many experiments. No, I’m just joking.
.
Do you have any additional or broader comments that you might like to share?

Iñaki Ruiz-Trillo (single-celled eukaryotes)
Work with people that are nice people, that are persons before scientists. Whether it’s a PhD, working for a supervisor or collaborators, whatever. You have to have people that you feel confident you can say a stupid thing. And you can laugh together and you can be interested together about the questions. Then everything will be much, much, much better.

Michalis Averof (shrimp)
Over the years, we have developed this idea that model organisms will reveal universal mechanisms, and that we can study most of biology through the model systems that we have chosen. But I am convinced that there is an enormous amount of biology that we are missing, if we rely only on the established models. There are biological phenomena which are not represented in this handful of organisms.
I see these like new continents of biology that are still unexplored. New model organisms will allow us to explore these. Of course, it’s going to be difficult, it’s going to take time, and it’s going to take development of tools. But that’s for me the major motivation for going into different systems, because I think there’s biology that we haven’t discovered yet.

Patricia Ornelas-García (Mexican cavefish)
The problem with non-model organisms is the conservation situation. Nobody will catch Mus musculus from the field, they have so many reproductive lines in captivity that they don’t have to. Non-model organisms are in the opposite situation. Most labs [working on them] want to have more wild lines, more related with what is really happening in the field. And if you have 200 labs working [on cavefish], imagine the impact that we can have on the natural population. We published, just at the beginning of the year, a paper [on] size estimation of the fish population in the caves, and it’s maybe around [a few] thousands of fish. And imagine, in the last 10 years, there have been around 200 fish extracted from the caves. So if you imagine a system that has to recover from 20% of the population being lost only because of scientific sampling, it’s problematic.
When you try to make researchers aware of the situation, [they] really believe that the main extinction drivers of this kind of population are not related with our sampling. Most of us really believe that it’s all global warming, or local people extracting water for drink. I’m very surprised, because normally you have to fight this kind of attitude outside the scientific [world].
Thus, it’s important to be part of the solution, not part of the problem. Maybe we should consider what we can do to solve the conservation situation. We need to be more aware about the impact of our research and do our best to guarantee the prevalence of this model for future generations. [Because] what makes these organisms amazing also makes them vulnerable, in a way.

András Simon (newt)
I was thinking about what makes an organism model or non-model? Is it a qualitative term, or quantitative? If enough numbers of researchers work on it, is it a model organism? If not, is it not a model organism? What is the definition? I would probably say that [the newt] is a model organism for regeneration, but it’s a non-model organism in the sense that not so many people work on it, although the community is growing.
.
We couldn’t have succeeded in our effort to make this compilation article without the generous time these researchers were willing to share with us, so our sincere thanks go out to András, Michalis, Paty, Cliff, and Iñaki. We are really hoping that this is just the beginning of our collective exploration of NMOs, and of greater interspecies research collaboration. The more we investigate, the more we can hope to understand how the amazing tree of life is built, interconnected, and how to best attempt to preserve it in all its messy glory.


 (3 votes)
(3 votes)