Retinal ganglion cell survival after optic nerve injury – what can we learn from the zebrafish?
Posted by Jeff Gross, on 14 December 2021
This project started in a somewhat roundabout way. Our lab (the Gross lab) has focused for many years on development of the retina, lens and optic cup, eye evolution, and more recently RPE regeneration. Several years ago, Taka Kuwajima, who is an expert in retinal ganglion cell (RGC) biology, and particularly axon regeneration, was hired into our Department. We share lab space and have a joint weekly meeting, and had wanted to work together on a project for some time but nothing quite got started. Then, in 2019, we had a fabulous MD/Ph.D. student, Si Chen, begin to work with us through a joint program between the University of Pittsburgh and Xiangya Hospital/Central South University in China. Si was interested in glaucoma, so we thought about what sort of RGC-focused project we could do together that would also finally get Taka and I collaborating.
RGCs in mice die rapidly after injury. The same is true in humans, and neither system can regenerate lost cells or axons if they are damaged. This is what leads to the loss of vision in glaucoma – once the RGC axons comprising the optic nerve are damaged, the cells begin to die and vision progressively deteriorates. Zebrafish have remarkable abilities to regenerate lost or damaged cells and tissues, and this is true for RGCs; when the axons of the optic nerve are damaged or even transected, zebrafish regenerate these projections and restore connections and function. Several labs are doing some really exciting work on axon regeneration including Lieve Moons, Ava Udvadia, and Dan Goldman. While the mechanism that facilitates RGC axon regeneration is a fascinating topic, what really interested us was a slightly different facet of zebrafish RGC biology coming from earlier studies where it was reported that ~75% of zebrafish RGCs were preserved after severe optic nerve injury, even to 7-weeks post-injury (Zou et al., 2013). Again, in mammals, the RGCs die rapidly after injury and to our knowledge, no one had followed up this observation that zebrafish RGCs stayed alive after completely severing their axons. We thought this would be a great project to bring our labs together and a great in vivo system to possibly discover something new about neuroprotection. Moreover, since there are no effective treatments to preserve RGCs in glaucoma, we thought this project might also have some exciting translational potential if we could discover genes and pathways that facilitated RGC survival after injury.
To get started, Si first repeated the experiments of Zou et al. (2013), observing exactly what they saw – RGCs did indeed survive, even after she completely severed the optic nerve! These are tough experiments and Si has amazing skills that enabled her to develop the surgical technique and rigorously reproduce the findings. With the model in hand, Si was then able to isolate RGCs and perform RNA-Seq to profile changes in gene expression after injury. This is a great hypothesis-generating experiment and indeed, she identified many genes and pathways that were altered by the injury. She decided to focus on Jak/Stat signaling as a proof-of-concept that this approach could yield interesting neuroprotective factors. Her results were striking; blocking Jak activity compromised RGC survival after injury. What was particularly exciting was how the project then dovetailed nicely with another interest in the lab – the role of immune responses during injury and regeneration. We had recently been studying innate immune responses during RPE regeneration (Leach et al., 2021) and leveraged some of what we had learned in that system to start to look at RGC survival. Si was able to show that blocking inflammation or depleting macrophages/microglia protected all RGCs after injury. The Jak/Stat pathway is activated in both RGCs and macrophages/microglia after injury, so we don’t yet know if activity is required in one cell type or both, and this is something for which we’d like to develop tools to answer.
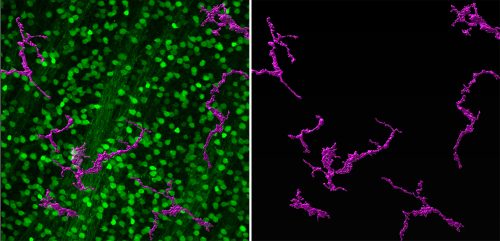
We’re excited to study some of the other genes and pathways identified in the screen and we’re also curious to see if there are more sensitive or resilient RGC subtypes. Our research will be assisted by the beautiful single cell atlas of zebrafish RGCs recently published by the Baier lab (Kölsch et al., 2021) and work from Nick Tran and colleagues, where they have identified resilient subtypes in mouse (Tran et al., 2019). A terrific new postdoc, Ashrifa Ali, has recently joined the lab and she plans to build off of these studies, using the zebrafish model to better understand RGC survival after injury. Hopefully, this will lead to the development of new therapeutics to treat glaucoma.
In the end, this was a really fun project that brought together a fantastic MD/Ph.D. student, Si Chen, with Taka and me to work together on something new for each of us. I love this part of basic science research; we have the best jobs in the world where we can come to work every day, ask exciting open-ended questions and then figure out creative ways to answer them. If you have a good idea and work hard, more likely than not, you will discover something new, and there aren’t many jobs that give this sort of freedom and reward. We have a lot more to do on this project, and certainly there will be a lot of ups and downs along the way, but I’m sure it will be a fun ride.
REFERENCES


 (1 votes)
(1 votes)