Retroviruses for Axolotl Research
Posted by Jessica Whited, on 1 February 2013
Many salamanders can regenerate limbs, and even a seven-year-old child appreciates exactly the reasons why this feat is so remarkable. How can an animal that has been living its life, using its leg full of muscles and bones and tendons and nerves every single day, suddenly grow a new one at some random time? When the leg first grew on the same salamander when it was just a tiny animal, we can imagine it followed a prescribed developmental program for its instructions and used a prescribed pool of progenitor cells for its construction. Once the limb tissues are already working, the cells are differentiated and doing their jobs, so how do they go back in time and create tissues anew? Scientific understanding of how limbs develop has progressed immensely over the last several decades, but understanding of how limbs can regenerate and why some animals do it better than others has remained elusive. This lag in understanding vertebrate limb regeneration on a molecular level stems from the fact that the two model systems with the most sophisticated tools for studying limb development—chick and mouse—do not naturally regenerate entire limbs as adults. Figuring out what the regenerative roadblocks are in chick, mouse, and human will be imperative for improving regeneration in species that do not do it well. However, it will be equally important—and perhaps fundamental for elucidating these roadblocks—to understand how animals that regenerate limbs remarkably well do it. This is why we are developing tools that allow for more precise molecular genetic and cell biological inquiry into axolotl limb regeneration.
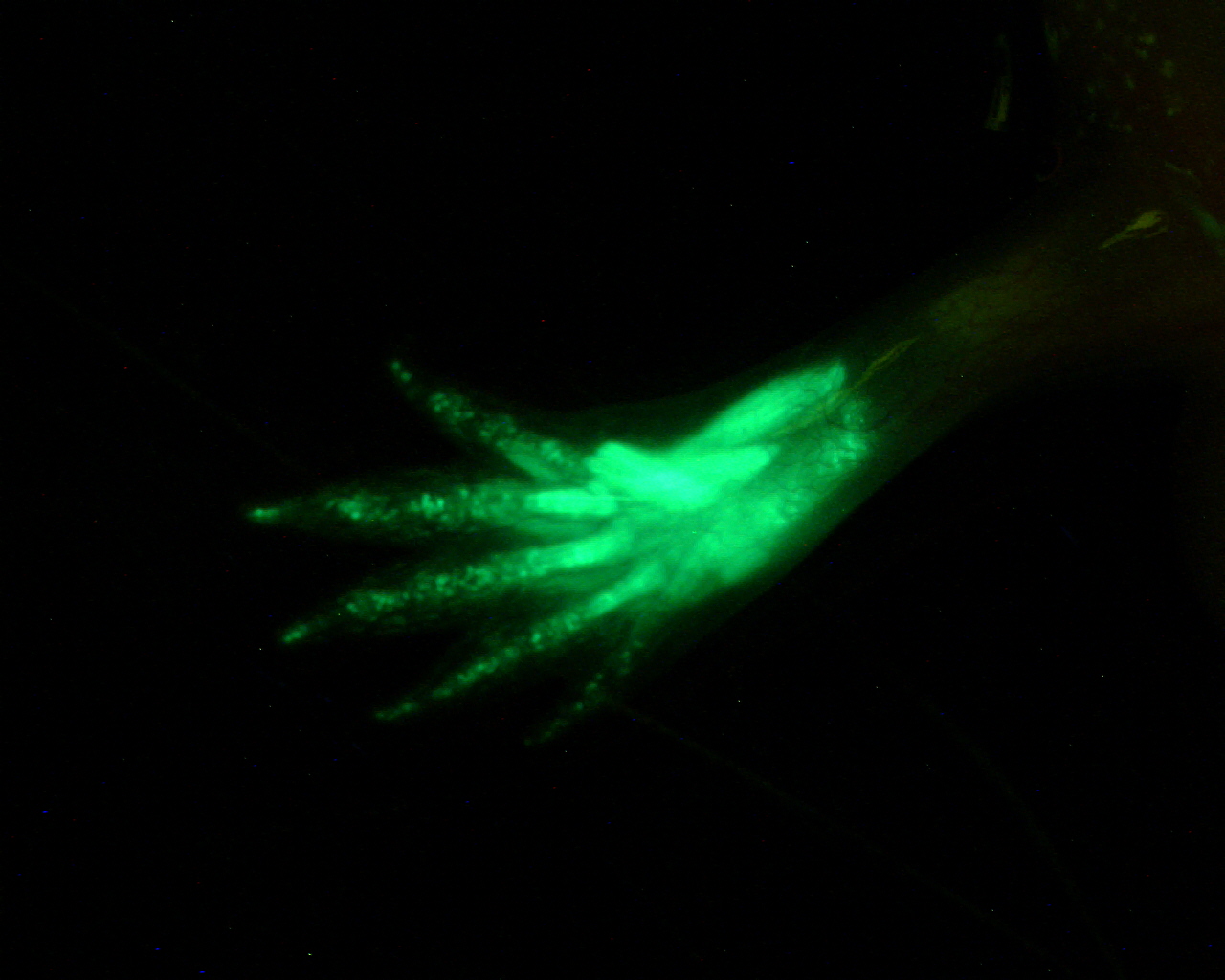
EGFP expression in a live animal. This axolotl hindlimb was amputated mid-femur. The blastema that formed was infected with EGFP-encoding retrovirus two weeks post-amputation, and the limb was allowed to fully regenerate. Cells descended from infected blastema cells express EGFP. Since the injection was limited to the blastema, cells proximal to the amputation plane do not express EGFP.
Two things a researcher might want to do when studying how salamanders regenerate limbs are tracking cells during regeneration and expressing introduced genetic elements to analyze their effects. In the past, labeling methods such as heavy isotopes, dyes, and electroporation of plasmid DNA have been used in regenerating salamander limbs. Electroporation of plasmid DNA has also been used to mis-express genetic elements. While studies using these methods provided key insights into, for example, which cells become mitotically active following amputation, all three of these methods suffer a similar drawback: the element gets diluted with each successive cell division, and in a regenerating limb, lots of cell division occurs. It was impossible to follow cells from the stump, through the blastema (group of relatively dedifferentiated cells that forms at the tip of the stump and will give rise to internal limb tissues in the regenerate), and into the new limb. Transplantation studies using tissues from permanently marked donors, such as animals induced to have a different ploidy or transgenic animals, have aimed to bypass these caveats, but transplantation is not ideal in many situations (for example, some tissues cannot be cleanly separated, and the procedure itself is obviously invasive and best done well before limb regeneration will be studied). Furthermore, donor tissue is relatively limited at this point to just a few genetic lines of axolotls constitutively expressing a fluorophore, and generation of novel animals by transgenesis is a lengthy and labor-intensive process. Expression studies using electroporated plasmid DNA were limited because even good electroporations may not lead to enough sustained expression to detect an effect. In our Development paper, we showed that retroviruses can infect regenerating axolotl limbs. These retroviruses are simply injected into limb tissue, and they can infect any mitotically active cell they encounter. Since the retroviral genomes integrate into host cells, they can be used to permanently express a label such as GFP, which allows for tracking cells during regeneration, opening the door to many future studies. Expression from the retroviral vectors is robust and using retroviruses might be a powerful way to finally address the consequences of misexpressing candidate genes during limb regeneration. Additionally, we showed that retroviral infections can in principle be targeted to specific cell types by targeting infections to vascular endothelial cells. This works by borrowing technology exploited by other researchers in the mouse. Axolotl and mouse cells do not make the receptor for a particular coat protein found on the surface of certain bird viruses. However, if axolotls or mice are made to express that receptor, they can become infected by viruses with these coats, and expression of the receptor can be confined to particular cell types by the researcher provided genetic elements for doing so (for example, a cell-type-specific promoter) exist. Hence, the retroviruses can also someday be targeted to a whole battery of different specific cell types in regenerating axolotl limbs once cell-type-specific promoters are found. These tools will allow for more precise control of labels and introduced gene expression during regeneration.
Figuring out how salamanders regenerate limbs won’t just satisfy the curiosity of the seven-year-old in us all, it also stands to someday dramatically improve the lives of the millions of people living with the consequences of limb amputation. With key risk factors such as diabetes and peripheral artery disease on the rise, limb loss is unfortunately becoming an even more common problem in places like the United States, giving scientists a call to arms when it comes to unraveling the mystery of limb regeneration.


 (10 votes)
(10 votes)