Roles of amino-acid transporters in the developing brain
Posted by Charlotte Campbell-Broad, on 11 October 2021

After a year of lockdowns and virtual classes at Bangor University, the opportunity to do a real lab project this summer at the Francis Crick Institute was definitely not one to miss. Under the patient supervision of Adrien Franchet and Sebastian Sorge in Alex Gould’s lab, I set out to explore the roles of some amino acid transporters during the development of the genetic model organism Drosophila.
This two month summer project was my first opportunity to gain hands-on experience doing hypothesis-driven science and to interact with many talented researchers at the Crick. As an undergraduate, my only previous exposure to fruit flies was from reading published papers but, right from day one, I got stuck in to the nitty gritty of Drosophila developmental biology and larval dissections.
The Gould lab are interested in figuring out how the neural stem cells of the developing CNS are so highly protected against environmental stresses such as nutrient restriction (NR) and hypoxia. This process is a key part of brain sparing, which involves sustaining the growth of the CNS at the expense of other organs such as adipose tissue. In mammals, brain sparing is commonly observed in neonates following intrauterine growth restriction. However, the key signalling and metabolic pathways underlying brain sparing are still unclear.
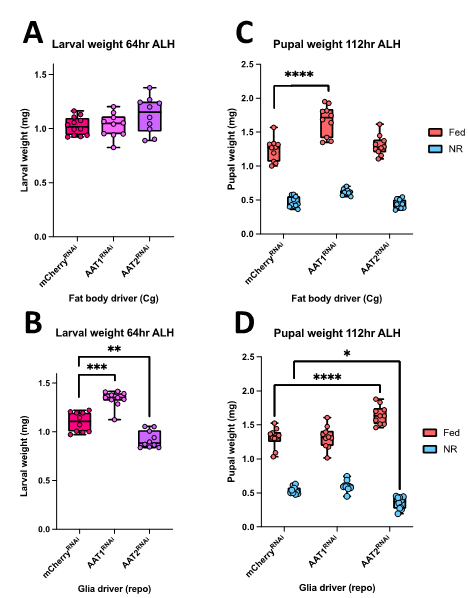
Amino-acids are key signals for growth and they are also critical for protein synthesis. The uptake of amino acids by tissues involves a large number of different amino-acid transporters and I set out to decipher whether two of these transporters (AAT1 and AAT2) are required in the neural stem cell niche (glia in Drosophila) or in adipose tissue (fat body in Drosophila) for CNS and body growth. My project stemmed from Adrien’s and Sebastian’s recent RNA interference (RNAi) screen of amino acid transporter candidates. I followed up two of their screen hits (AAT1 and AAT2) using UAS-RNAi knockdowns lines crossed with Gal4-driver lines specific for glia (repo-Gal4) or fat body (Cg-Gal4). The goal was to measure the phenotypic effects of these cell-type genetic manipulations during standard fed development and also during severe NR on an agar-only diet. Phenotypes were measured for larval and pupal weights using an accurate microbalance. I also quantified CNS phenotypes from confocal microscopy images by measuring CNS area and also neural stem cell (neuroblast) proliferation via the incorporation of a labelled nucleotide analogue (EdU).
I found that RNAi knockdowns of either AAT1 or AAT2 produced more severe phenotypes in glia compared to fat body (Figure 1). Hence, larval pupal and adult weights were largely normal with the fat body knockdowns (Figure 1A, 1C). However, both glial knockdowns gave modest changes in body weight at the larval stage but, by the pupal stage, these only remained significant for AAT2 (Figure 1B, 1D). I also noticed that glial knockdown of AAT2 eventually resulted in adult lethality, shortly after eclosion, with flies displaying very severe locomotor defects.
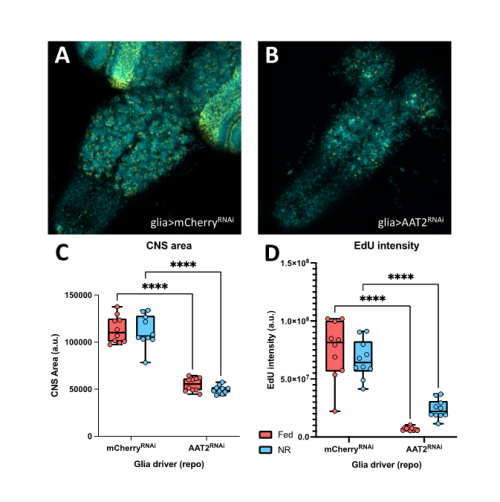
In a parallel set of experiments, I investigated the effects of the AAT2 knockdowns on the growth of the developing CNS and on the proliferation of neural stem cells. To do so, I dissected brains from fed larvae and from larvae exposed to one day of NR. I then performed an in vitro EdU incorporation assay as an indicator for neuroblast progression through S-phase of the cell cycle. I found that the fat body manipulations had no significant effect on CNS size or on neuroblast proliferation. In contrast, the glial manipulations revealed that AAT2 is required in glia for proper growth of the larval brain, as the CNSs of repo-GAL4; AAT2RNAi larvae were strongly reduced in size and likewise the EdU incorporation was much lower than genetic controls (Figure 2A, 2B). This glial requirement for AAT2 for neuroblast proliferation occurred in both fed and NR larvae (Figure 2C, 2D). Thus, in conclusion, my project has revealed a constitutive function in glia for the amino acid transporter AAT2 during both normal CNS growth and brain sparing. It will be important in future to explore whether AAT2 is required in the surface glia of the blood-brain barrier or in the internal cortex glia that surround neuroblasts and their daughter cells. Equally importantly, it will be interesting to identify which specific amino acids are transported by AAT2.
Overall, this fascinating project has given me a first taste of biological research at the bench and has also allowed me to develop critical thinking and data processing skills. I am indebted to Adrien Franchet and Sebastian Sorge for their fantastic direction, and to Alex Gould and all of his lab for their encouragement throughout. I would also like to thank the Francis Crick Institute for hosting me and the Medical Research Foundation Rosa Beddington Fund for supporting my project and allowing me to contribute to this captivating field of research.


 (2 votes)
(2 votes)
Fascinating project. Well done