Shining a Light on Adult Neural Stem Cells
Posted by Alina Marymonchyk, on 2 October 2021
Adult Neural Stem Cells (NSCs) have a remarkable capacity to produce new neurons and glia cells that integrate into pre-existing neural networks. Adult NSCs are found in all mammals, including humans, giving us hope of using the pool of adult NSCs to replace damaged/diseased cells affected by neurodegenerative disorders and brain trauma.
NSCs can be found in two states, quiescent (resting) and active. Activated NSCs eventually divide to generate new cells. However, while in rodents NSCs are activated and produce neuronal progenitors, in humans they are found mostly in the quiescent state. Given this, the promise of using the endogenous capacity of NSCs to repair brain damage cannot be fully realized without understanding the mechanisms that regulate NSC quiescence and activation. Indeed, the uncontrolled proliferation of NSCs may result in the formation of tumours, whereas the enhancement of NSC quiescence may restrict their regenerative capacities. It is thus essential to understand the mechanisms that control the transition between the quiescent and active states in order to develop strategies to fine-tune NSC activity.
We recently published a paper (Gengatharan et al., Cell, 2021) that shed light on the mechanisms that regulate the transition of NSCs from the quiescent to the activated state in freely behaving animals. This paper addresses several questions related to NSC physiology.
Do animal behaviour and environmental factors influence the transition of NSCs from the quiescent to the active state?
Although we have a growing understanding of various pro-proliferative and pro-quiescent molecular cues, it is unclear how NSC proliferation can be reliably and cyclically regulated throughout every day of an animal’s life and how the environment in which animals live affects NSC activation and division. What happens in the life of an animal that occurs every single day independently of anything else? The day/night cycle! We used advanced imaging techniques to record NSC division in freely behaving animals and showed that ~70% of divisions occur during the day and only ~30% at night. The “shining of light” induces NSCs activation, while the lack of light during the night switches some of the cells to the quiescent state.
Melatonin is front of mind when talking about the day/night cycle. Melatonin levels increase in our body at night as they do in nocturnal animals such as rodents. Such a big systemic change appears to regulate the delicate balance between proliferation and quiescence every day of an animal’s life, preventing excessive NSC proliferation.
However, it is undoubtably not the only an environmental change and a signaling molecule that affect the state of NSCs. Imaging of freely behaving animals will allow us to correlate changes in proliferation with animal behaviour and the surrounding environment. It can also be used to uncover other systemic factors that increase or decrease NSC proliferation and to decipher the molecular mechanisms underlying these changes.
What are the intracellular pathways in NSCs on which various extracellular signals impinge to modulate affect cell proliferation or quiescence?
How NSCs can decode and integrate pro-proliferative and pro-quiescent signals to decide whether to become active and divide or to remain quiescent? Although signals can be very different in abundance, time scale, signaling cascade, etc., would it be possible to combine all these signals into one overarching mechanism?
We used in vivo and ex vivo imaging to show that calcium dynamics serve to translate micro-environmental changes and various inputs into changes in the state of NSCs. Calcium is a versatile cellular messenger and many of the parameters of its dynamics can be influenced. We showed that quiescent and active cells display different frequencies and amplitudes of calcium events and have different intracellular loads of calcium ions. Most cells are quiescent and display a high frequency of calcium events and low intracellular calcium levels. This calcium signature is maintained by pro-quiescent signals. In response to pro-proliferative factors, the frequency of calcium events decreases and intracellular calcium levels increase, triggering NSC activation.
Can calcium alone trigger a change in the state of NSCs or is it merely reflection of the state of NSCs?
We used optogenetic tools to mimic the calcium pattern of quiescent cells in animals. Constant daylight was used to stimulate NSC activation. Interestingly, this optogenetic stimulation decreased NSC proliferation even below the baseline level. This fascinating result showed that the transition from one state to another can be manipulated and that increased proliferation can be reversed using the universal nature of calcium signals.
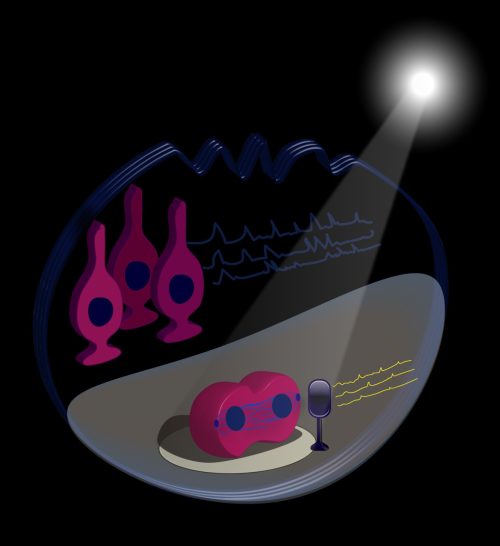
To summarize, we designed an illustration (Figure 1) featuring a stem cell on a performance stage with a light shining on it. It sings solo with low frequency calcium traces and divides. It is accompanied by a backstage chorus of quiescent cells (which are the majority of stem cells) that sing with high frequency calcium traces. “Shining a light on stem cells” is thus also a figurative expression of NSC division in live animals, where they play a role on stage and are watched by an audience of researchers.
–
Gengatharan, A., Malvaut, S., Marymonchyk, A., Ghareghani, M., Snapyan, M., Fischer-Sternjak, J., Ninkovic, J., Götz, M. and Saghatelyan, A. (2021). Adult neural stem cell activation in mice is regulated by the day/night cycle and intracellular calcium dynamics. Cell 184, 709-722.e713. doi:10.1016/j.cell.2020.12.026


 (2 votes)
(2 votes)