From our sister journals – June 2016
Posted by the Node, on 29 June 2016
Here is some developmental biology related content from other journals published by The Company of Biologists.

Cartilage development downstream of Notch
 Notch signalling regulates various aspects of vertebrate cartilage development, and Hilton and colleagues now investigate the role of the Notch effectors HES1 and HES5. These transcription factors suppress chondrogenesis and promote chondrocyte hypertrophy, with some overlapping and some distinct functions.
Notch signalling regulates various aspects of vertebrate cartilage development, and Hilton and colleagues now investigate the role of the Notch effectors HES1 and HES5. These transcription factors suppress chondrogenesis and promote chondrocyte hypertrophy, with some overlapping and some distinct functions.
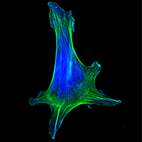
Scribble promotes proliferation
The scaffolding 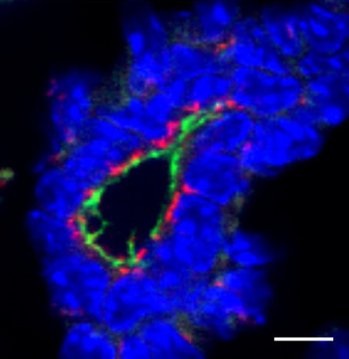 protein Scribble is critical in establishing apical-basal polarity during epithelial development. Muthuswamy and coworkers now show that during pregnancy, Scribble has an unexpected role in promoting cell proliferation during alveologenesis, potentially via keeping the prolactin receptor at the cell surface.
protein Scribble is critical in establishing apical-basal polarity during epithelial development. Muthuswamy and coworkers now show that during pregnancy, Scribble has an unexpected role in promoting cell proliferation during alveologenesis, potentially via keeping the prolactin receptor at the cell surface.
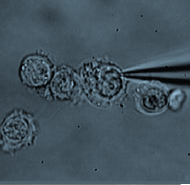
A molecular pathway to haptotaxis
 Haptotaxis is directional cell migration in response to a gradient of substrate-bound cues. Bear and colleagues investigate the cellular and molecular basis of haptotaxis using microfluidic chambers, and show that differential actin and lamellipodial dynamics, regulated by Arp2/3 and its upstream regulators, contribute to the process.
Haptotaxis is directional cell migration in response to a gradient of substrate-bound cues. Bear and colleagues investigate the cellular and molecular basis of haptotaxis using microfluidic chambers, and show that differential actin and lamellipodial dynamics, regulated by Arp2/3 and its upstream regulators, contribute to the process.

A new way to generate photoreceptor-like cells
 De novo generation of photoreceptor cells is therapeutically promising for patients with retinal degenerative diseases. Seko and colleagues describe efforts to directly reprogram blood cells into photoreceptor-like cells using the CRX transcription factor. This method may provide a cost-effective alternative to induced pluripotent stem cells for personalised drug screening and disease modelling [OA].
De novo generation of photoreceptor cells is therapeutically promising for patients with retinal degenerative diseases. Seko and colleagues describe efforts to directly reprogram blood cells into photoreceptor-like cells using the CRX transcription factor. This method may provide a cost-effective alternative to induced pluripotent stem cells for personalised drug screening and disease modelling [OA].
Polyamines in pigmentation
Zebrafish  pigmentation is an established model system for developmental patterning. Irion and colleagues now identify a new player in pigmentation: the polyamine spermidine. Mutations in the spermidine synthase gene leads to loss or interruption of the dark stripes of the flanks and fins [OA].
pigmentation is an established model system for developmental patterning. Irion and colleagues now identify a new player in pigmentation: the polyamine spermidine. Mutations in the spermidine synthase gene leads to loss or interruption of the dark stripes of the flanks and fins [OA].

Picking the right model
In his Editorial, Leonard Zon explores how complementing his lab’s primary model organism – the zebrafish – with other model systems helped in the translation of research to the clinic , and the importance of collaboration and infrastructure [OA].
Distinct cellular contributions to muscle repair
Hughes and colleagues 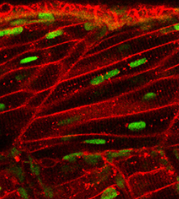 identify stem cell diversity in the wound healing response of zebrafish muscle, and propose that pax7a-expressing cells initiate de novo fibre formation, while pax7b-expressing cells promote fibre growth [OA].
identify stem cell diversity in the wound healing response of zebrafish muscle, and propose that pax7a-expressing cells initiate de novo fibre formation, while pax7b-expressing cells promote fibre growth [OA].
Linking neural crest cell migration to craniofacial disorders
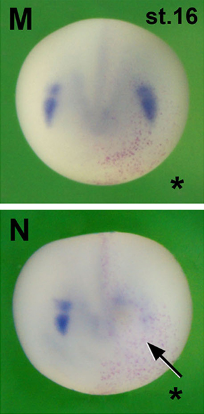 Pera and co-workers use Xenopus to model Musculocontractural Ehlers–Danlos syndrome, which is caused by mutations in dermatan sulfate enzymes. The craniofacial abnormalities associated with the disorder may arise from defective migration, rather than specification, of neural crest cells [OA].
Pera and co-workers use Xenopus to model Musculocontractural Ehlers–Danlos syndrome, which is caused by mutations in dermatan sulfate enzymes. The craniofacial abnormalities associated with the disorder may arise from defective migration, rather than specification, of neural crest cells [OA].


 (2 votes)
(2 votes)