The role of WNT signalling upon injury in intestinal stem cells
Posted by Amie Stott, on 13 November 2024

Over the summer, I had the opportunity to conduct research in Vivian Li’s Lab, focusing on the role of WNT signalling in the response of intestinal stem cells to injury, under the expert guidance of Dr. James Wilmouth Jr. As a medical student, I am deeply appreciative of the opportunity to gain hands-on experience in fundamental research through undertaking a project over the course of two months. I am truly inspired by the continuous innovation at the Francis Crick Institute, driven by a diverse community of committed scientists who embrace collaboration and interdisciplinary approaches.
One research axis within the Li Lab focuses on how canonical WNT signalling maintains intestinal stem cells (ISCs) and influences cell fate decision. The gut epithelium can be organised into an architecture of crypts and villi (Fig 1A). Crypts are found at the base, extending into the villi towards the gut lumen. ISCs are located within the crypt and responsible for driving renewal of the gut epithelium every 3-5 days during homeostasis. High WNT signalling in the crypts maintains ISC homeostasis before decreasing along a gradient towards the villi. Publications have demonstrated that whilst inhibiting WNT signals causes degeneration, WNT overactivation induces adenoma formation. Therefore, fine-tuned WNT signalling is crucial for maintaining ISC homeostasis.
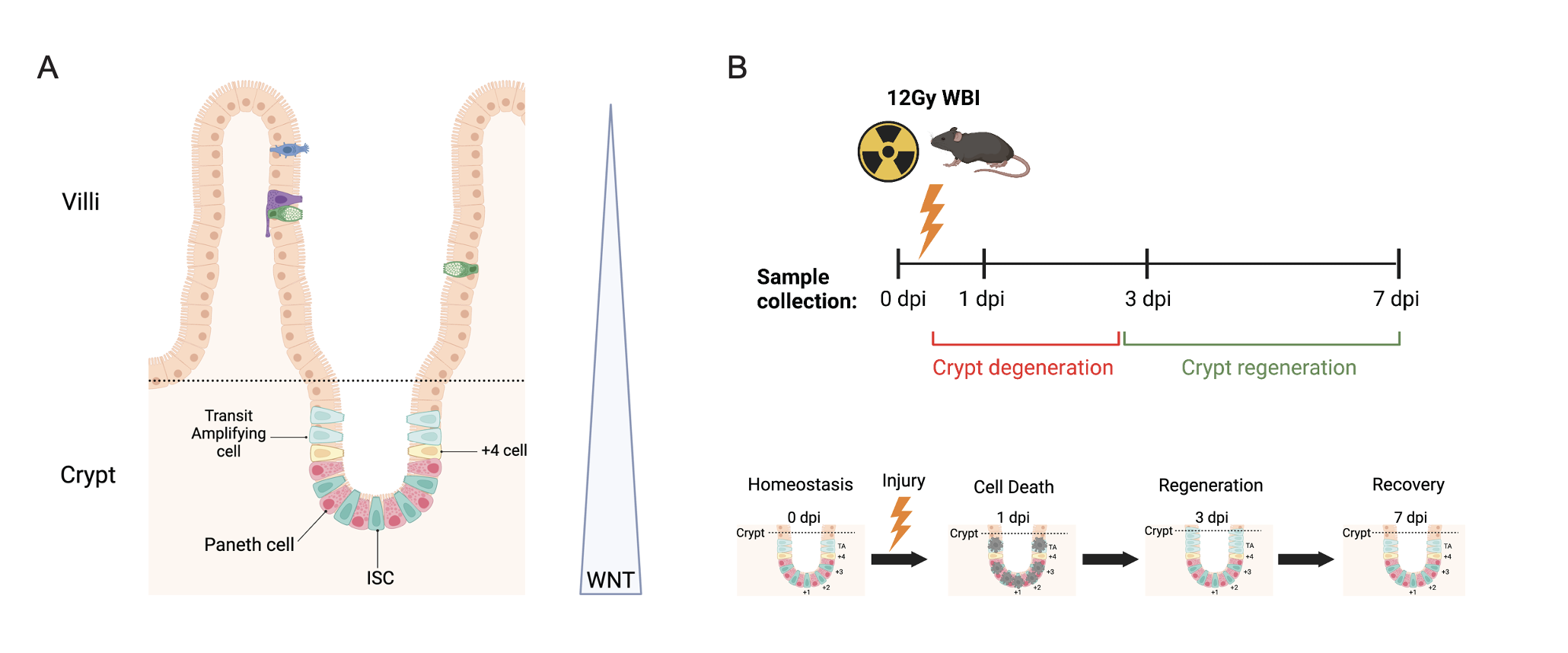
For this project, I examined the role of WNT signalling in ISCs during injury-induced regeneration. We utilized whole-body irradiation to simulate ISC injury-induced regeneration in mice. This model results in crypt degeneration at 1-day post-irradiation (dpi), followed by regeneration at 3 dpi, and recovery by 7 dpi (Fig 1B). Intestinal tissues were harvested for crypt isolation at four time points: 0 dpi (pre-irradiation control), 1 dpi, 3 dpi, and 7 dpi. These samples were then used to investigate three transcriptional signatures throughout the regenerative process: Classical Intestinal Stem Cell (ISC), Revival Stem Cell (RevSC), and WNT target genes.
I performed quantitative PCR (qPCR) to analyse the temporal changes in gene expression during the regenerative process (Fig 2). Results indicated that classic ISC markers were downregulated at 1 dpi following injury and recovered to near homeostatic levels by 7 dpi. RevSC markers showed an induction at 1 dpi, followed by a decline toward baseline levels by 7 dpi. Interestingly, WNT target gene expression remained relatively stable throughout the 7-day period.
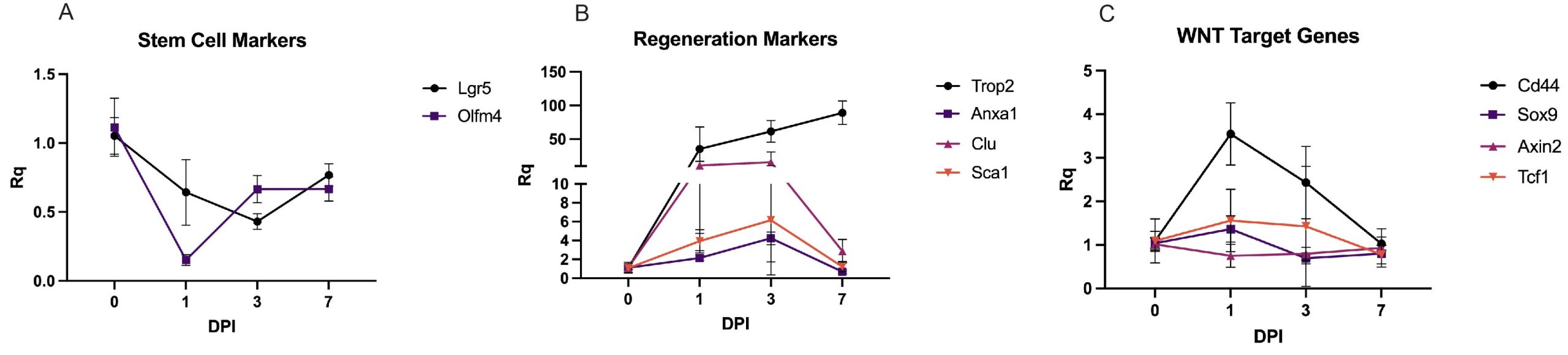
These initial results suggest that both Yap-driven RevSC signature and certain WNT targets are expressed during injury-induced regeneration. This contradicts the current understanding, which proposed an antagonistic relationship between YAP and WNT during ISC regeneration up on injury. To further investigate the co-expression of RevSC markers and WNT in ISCs, we utilised immunohistochemistry (IHC) and flow cytometry of WNT reporter mice in the irradiation induced regeneration model (Fig 3).
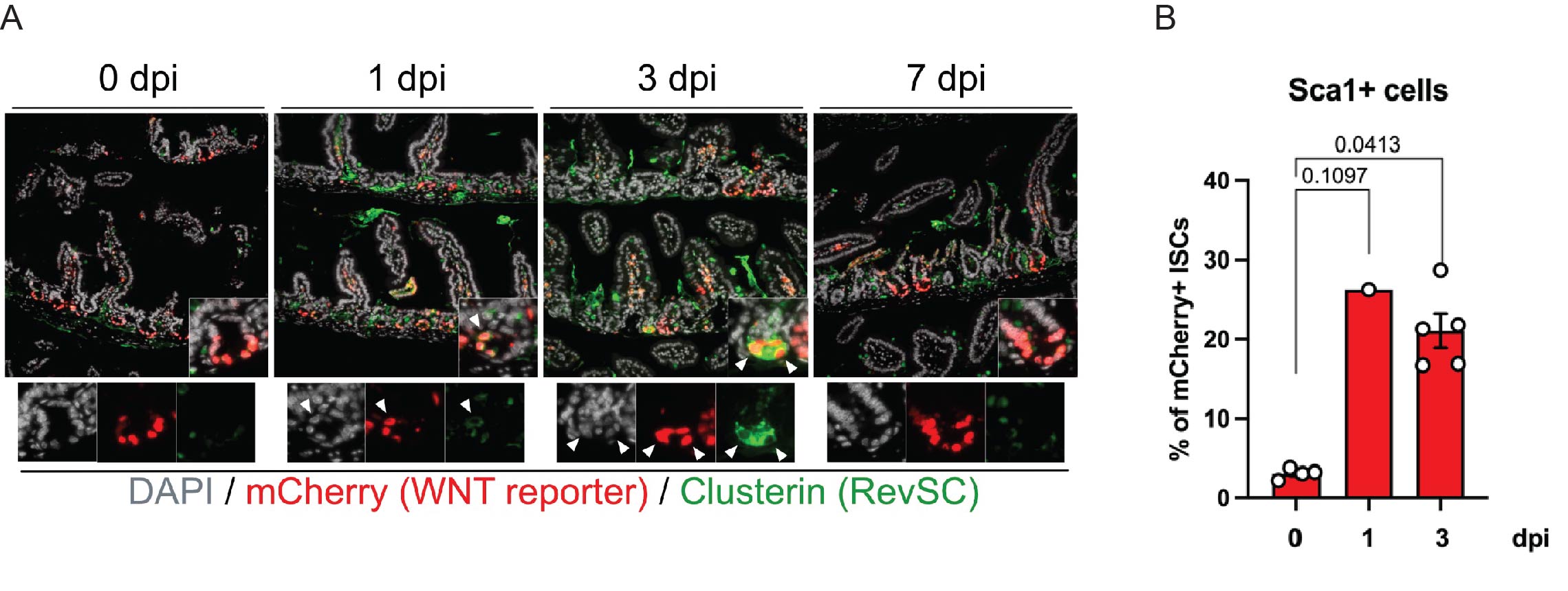
To spatially characterise WNT-high cells in the crypts co-expressing the Revival Stem Cell (RevSC) signature, I conducted IHC using a TCF/Lef:H2B;mCherry reporter system. The reporter has Tcf/Lef binding sites which drive the expression of the H2B:mCherry fluorescent protein, enabling visualisation of active WNT signalling. I observed colocalisation of mCherry and Clu (RevSC marker) at 1dpi, which was more pronounced at 3dpi (Fig 3A). These results suggest that WNT-high ISCs express RevSC markers during regeneration. In order to quantify how often this happens during regeneration, I utilised flow cytometry. By gating for mCherry+ ISCs (WNT-high ISCs), I found there was a significant increase in the percentage of mCherry+ ISCs expressing the RevSC marker Sca1 at 3dpi (~20%) compared to 0 dpi (~3%) (Fig3B).
In conclusion, my project demonstrated that a proportion of WNT-high ISCs co-express RevSC markers during injury-induced regeneration. Although preliminary, these results highlight that it is necessary to further investigate the role of YAP in the interplay between WNT signalling and the RevSC signature in ISCs during regeneration. This work would provide clarity into which signals dictate ISC survival during regeneration.
This project has provided me with invaluable insight into the scientific process. I have learned advanced techniques at the Li lab, including organoid culture and maintenance. I would like to thank my supervisor, James, for his exceptional mentorship in building my critical thinking, guiding my experiments, and supporting my data analysis and interpretation. I would also like to extend my thanks to the Francis Crick Institute and the Medical Research Foundation Rosa Beddington Fund for their generous support, which has enabled me to contribute to this exciting field of research.


 (2 votes)
(2 votes)
This is such a lovely insight into the research process at the Crick! <3