When a medical doctor steps into a developmental biology lab to study the mechanics of human embryo development
Posted by Jean-Léon Maître, on 29 August 2024
In the paper “Mechanics of human embryo compaction”, Jean-Léon Maître and colleagues mapped the mechanical forces controlling compaction during human embryogenesis. Find out more about the behind the paper story from corresponding author Jean-Léon Maître and first author Julie Firmin, who is a medical doctor specializing in Assisted Reproductive Technology.
How did the project start?
Jean-Léon: In 2017, shortly after I had started my team at Institut Curie, Edith Heard, who was the director of my department, introduced me to Catherine Patrat with whom she had worked a few years before. Catherine heads the largest IVF clinic in France at Hospital Cochin, which is only a few minutes’ walk from Curie. Fortunately, Catherine got interested in our research and we decided to start with the simplest possible project about human embryo mechanics, which is compaction. It was then only a matter of getting the permit, money and the right person for the project. Aaaaand, it was the end of 2019 🙃.
Julie, what led you to join Jean-Léon’s lab?
Julie: I am a medical doctor, specialized in Assisted Reproductive Technology (ART). ART is still a relatively recent medical discipline—the first IVF births date back to 1978—and success rates after embryo transfer are only around 30%. Meanwhile, a recent WHO report estimates that one in six people is or will be affected by infertility at some point in their lives. My attraction to research stems from the conviction that better understanding the embryo is crucial for improving ART.
I discovered the work conducted by Jean-Léon on preimplantation embryos during my second year of Master degree, which I was pursuing alongside my medical studies. At that time another student, co-supervised by Jean-Léon and Professor Catherine Patrat, head of the ART department at Cochin Hospital in Paris, was studying compaction of human embryos using time-lapse images of embryos obtained after IVF. Using quantitative measurements inspired by knowledge of mouse embryo mechanics, the student aimed at identifying more robust parameters than the qualitative ones routinely used in ART laboratories to select which embryo to transfer. This piqued my interest in the basic research being conducted in Jean-Léon’s laboratory and motivated me to learn the biomechanical approaches they use to study embryonic development.
Following an internship as a medical resident in Professor Patrat’s ART laboratory, during which I was able to develop my skills as an embryologist in a human IVF unit, I had the opportunity to join Jean-Léon’s team full-time at the end of 2019 as a PhD student.
What was known about the mechanics driving human embryo morphogenesis before your work? Since the advent of in vitro culture, the morphology of the human embryo has been observed and described in numerous studies. In fact, along with cell number, the morphology of the human embryo is a primary determinant in clinicians’ assessment of the implantation potential of human embryos. Despite the importance of human embryo morphology for its development, we know very little about the mechanisms responsible for shaping the human embryo and can only extrapolate what we know from model organisms.
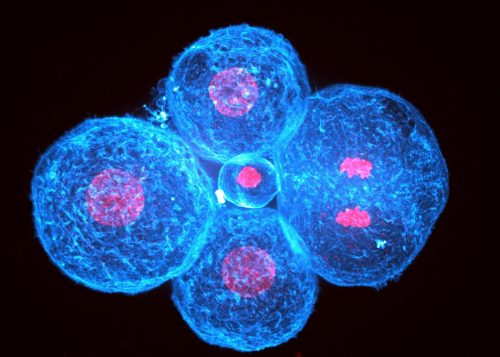
Human embryonic development starts with cleavage divisions without much change in the morphology of the blastomeres. Human morphogenesis starts with compaction and is the first step leading to the formation of the blastocyst, which will implant the embryo into the maternal uterus. During compaction, blastomeres come closer together, forming a tighter structure. The compaction process was long thought to be driven by increased cell adhesion via modifications of the calcium-dependent cell-cell adhesion machinery, particularly CDH1-dependent adhesion, since removing extracellular calcium prevents CDH1 binding and causes embryos to decompact. However, the possibility that cells could also use cell contractility to pull themselves together, as suggested by 20 years of study of animal morphogenesis, had not been explored in human embryos.


Can you summarise the key findings in a paragraph?
To measure the forces responsible for human embryo compaction, we used micropipette aspiration. This technique is mostly non-invasive, compatible with long-term development and we had used it extensively with mouse embryos, i.e. we knew it would work right away with precious donated human embryos. Micropipette aspiration reveals increased forces at the cell surface and stable forces at cell-cell contacts throughout compaction. Inhibition of cell contractility or cell-cell adhesion shows that only contractility controls the large forces present at the cell surface. Therefore, as observed in compacting mouse embryos and during morphogenetic processes of other animals, cell contractility is responsible for pulling cells together and plays an important role in shaping the embryo. Interestingly, some human embryos show compaction defects, which we can relate to abnormal contractile forces. Together, these experiments further confirm the key role of contractility in animal morphogenesis and identifies defective contractility as a potential cause for failed human embryo development.
How do the mechanics of mouse and human embryo morphogenesis differ?
At the scale of the embryo, it is fascinating to see how embryonic structures can be both conserved between mice and humans while still have their own unique shapes. For example, although both species form a blastocyst, the human blastocyst has an inner cell mass that protrudes more into the blastocoel than that of the mouse. This strongly suggests that the mechanical properties of these two species differ.
Considering compaction, the shape changes in mouse and human embryos are quantitatively the same: both increase the external contact angles formed by cell-cell contacts from ~80 to ~150°. Naïvely, one could think (JL: as I definitely did), that identical shape changes in related species would result from the same mechanical changes. What surface tension measurements reveal is that mouse embryos compact by reducing their tensions at cell-cell contacts and doubling their tensions at their free interfaces whereas human embryos rely exclusively on a four-fold increase of tension at their free interfaces. Mechanically speaking, the mouse embryo adopts a more efficient way of compacting than human embryos do. We do not know how mechanical efficiency might be related to metabolism or any kind of evolutionary fitness but we think that mechanical measurements can provide new and original insights into our understanding of the evolution of morphogenesis. For example, here we see that the same morphogenetic movement in related species does not necessarily rely on the same mechanical strategy.
What are the implications of your findings on the understanding of human embryos compaction failure during IVF?
After IVF, only 40% of embryos undergo complete compaction. The remaining embryos may partially compact, containing cells that remain loosely attached to the compacted part of the embryo. These cells are suspected to be aneuploid and their exclusion was proposed to serve as some kind of protection or repair mechanism that would prevent unfit cells from being part of the embryonic tissue. However, we did not know how such mechanism would work. We now know that excluded cells are systematically less strong than their compacting neighbors, which outcompete the weak cells for position in the inner cell mass that will make all embryonic tissues (see https://doi.org/10.1038/nature18958 for more on this).
Also, our measurements reveal the very high tension at the surface of human embryos, which we think is also connected with cell fragmentation, a deleterious process that is common in human embryos. Indeed, in another study from the lab we found that increased tension in mouse embryos causes them to fragment (https://doi.org/10.15252/embj.2023114415).
Julie, were there any particular result or eureka moment that has stuck with you?
Julie: When Jean-Léon measured surface tension of mouse embryos, the question of measuring excluded cells did not arise because compaction in mouse embryos is always perfect and complete. Therefore, we had not initially planned to look into excluded cells from partially compacting human embryos. From the start, I measured all cells of all embryos, regardless of their quality. Jean-Léon admitted that he would not have measured all of these cells since he cared mostly about normal development but I could not resort myself to give up on any of these embryos! After accumulating and analyzing the data from all embryos, good or bad looking ones, we could see the distinct mechanical signatures linked to abnormal compaction in human embryos. That was nice!
And the flipside: any moments of frustration or despair?
Julie: There were some moments of frustrations with inhibitors or antibodies that would not work with human embryos in the same way as they did with mouse embryos. Since donated human embryos are very precious, we could not troubleshoot too much and it was always stressful to plan experiments and decide whether it was worth spending embryos to troubleshoot or if it was better to give up.

What’s next for you, Julie?
Julie: I am now what’s called a Hospital-University Assistant. This involves IVF work at Cochin Hospital in Paris, and teaching at the University of Paris Cité. Also, I continue to do research by collaborating with Jean-Léon and his team.
I am delighted to have these three roles, as they allow me to continue my research in developmental biology, to take care of patients suffering from infertility, and to contribute to the education of future professionals who will, in turn, take care of these patients.
And JL, where will this story take the lab?
Jean-Léon: I am very excited to look into more aspects of the mechanics of human embryos. For all our biophysical approaches in the lab, we always keep in mind that we may be interested to try them on human embryos. Therefore, a new measurement needs to be as little invasive as possible and to provide a metric with a physical unit so that we can compare human embryos to those of other species. We have a couple of new approaches in the pipeline that hopefully will be used on human embryos in the coming years.
Reference list
Firmin J, Ecker N, Rivet Danon D, et al. Mechanics of human embryo compaction. Nature. 2024;629(8012):646-651. doi:10.1038/s41586-024-07351-x
Maître JL, Turlier H, Illukkumbura R, et al. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536(7616):344-348. doi:10.1038/nature18958
Pelzer D, de Plater L, Bradbury P, et al. Cell fragmentation in mouse preimplantation embryos induced by ectopic activation of the polar body extrusion pathway. EMBO J. 2023;42(17):e114415. doi:10.15252/embj.2023114415


 (No Ratings Yet)
(No Ratings Yet)