The people behind the papers – Anjali Rao & Carole LaBonne
Posted by the Node Interviews, on 9 August 2018
The neural crest is a progenitor population with the capacity to contribute to all vertebrate germ layers. The transcription factor and signalling pathway activity underlying this remarkable pluripotency have been well studied, but the role of the epigenetic state is less well understood. A new paper in Development examines the role of histone acetylation in regulating the neural crest’s remarkable pluripotency. We caught up with authors Anjali Rao and Carole LaBonne, Erastus O Haven Professor of Life Sciences at Northwestern University, to find out more about the story.

Carole, can you give us your scientific biography and the questions your lab is trying to answer?
CLB I have always been in love with the beauty of embryonic development, and the elegance of developmental decision-making. I did my PhD work at Harvard studying germ layer formation; specifically the role of FGF signalling in mesendoderm formation. During my graduate work I learned about the neural crest and I knew that was what I wanted to work on as a post-doc. It is an absolutely fascinating cell type, and this year is the 150th year of its discovery by Wilhelm His. Acquisition of the neural crest drove the evolution of vertebrates because these stem cells contribute a myriad of novel structures to the basic chordate body plan. Another interesting feature of these cells is that they are migratory and invasive; they need to actively disperse throughout the embryo to the places where they will form those structures. The mechanisms that they use to do this have been co-opted by cancer cells to mediate metastasis, so we can actually learn important things about tumor progression by studying the neural crest.
Two of Carole’s early papers (in Development!), from her PhD and postdoc
After my post-doc at Caltech I moved to Northwestern, where my lab has continued to study various aspects of neural crest development, including the signalling pathways and GRN components that control the stem cell attributes, behaviour and lineage decisions of these cells. More recently we have also been studying the pluripotent cells of the early blastula embryo. We do most of this work in Xenopus, which is a fantastic system for asking questions about early vertebrate development. Xenopus is closely related to mammals but the embryos develop rapidly and externally in simple saline solution, and the large (~1.2mm) size of the embryos facilitates isolation of explants or single cells. These embryos also provide copious materials for genomic and proteomic studies, which we are doing a lot of these days. Xenopus embryos are ideal for rapid gain/loss of function studies, including CRISPR-mediated genome editing, and the fate of every early embryonic cell has been mapped. We are very fortunate to have a fantastic resource center for this model, the National Xenopus Resource (NXR), that is located at the MBL in Woods Hole MA. The NXR generates and distributes transgenic lines and CRISPR mutants to the community, and also holds training workshops.
Anjali, how did you come to join the LaBonne lab, and what drives your research?
AR I have always been interested in understanding the regulation of stem cell maintenance. Before starting my PhD, I was working in a lab that studied the regulation of kidney stem cells during kidney fibrosis. Often diseases are caused by the misregulation of developmental pathways, and I hence got interested in studying the regulation of stem cells during embryonic development. The LaBonne lab was the perfect fit. Early during my PhD, our lab proposed a new model for the genesis of the neural crest that suggested that these cells arise due to a retention of stem cell potential, and we became really interested in identifying the mechanism for this. In particular, I was tasked with identifying chromatin remodelers required for neural crest stem cell maintenance, and characterizing the epigenetic changes that take place during the process of neural crest formation.
What led you to become interested in the epigenetic regulation of neural crest and blastocyst pluripotency?
CLB A few years ago we realized that neural crest cells and pluripotent blastula cells share a remarkable number of gene regulatory components. In particular, many of the transcription factors that we had long studied in the neural crest are first expressed in, and play essential roles in, blastula stem cells. That led us to hypothesize that neural crest may have arisen (and thus vertebrates evolved) via retention of features of these early pluripotent cells, even as neighbouring cells became lineage restricted. We began actively studying the shared features of these two cell types at the level of signalling pathways and transcription factors. For example some of our most recent work has focused on FGF-mediated Map Kinase signalling, which is essential for maintaining the developmental potential of both cells types. This was fun for me because it brought me full circle back to my graduate studies.
There is growing literature on the roles of epigenetic readers, writers, and erasers in controlling stem cell attributes and lineage decisions in both cultured ES cells and embryos. Thus, it was clear that we would need to examine conserved and divergent roles for these factors, and epigenetic mechanisms more generally, in neural crest and pluripotent blastula cells. We are finding interesting roles for a number of epigenetic regulatory factors, and so Anjali’s HDAC paper is just the tip of that iceberg.
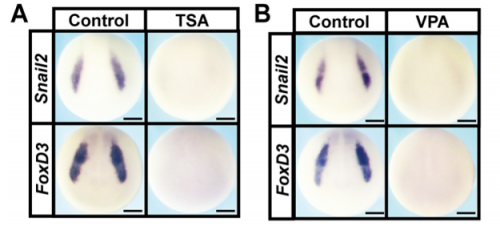
Can you give us the key results of the paper in a paragraph?
AR & CLB We found that Histone Deacetylase (HDAC) activity is essential for neural crest formation, both in whole embryos and explants of pluripotent cells. HDAC activity is also required for the pluripotency of blastula stem cells. Importantly, both cell types are characterized by low levels of histone acetylation, highlighting another shared feature of these cell populations, and HDACs function to maintain this state. Interestingly, we found that blocking HDAC activity in blastula-derived cells results in aberrant expression of markers of multiple lineages, and prevents the cells from committing to any lineage. Finally, we found that increasing HDAC activity can enhance the reprogramming of cells to a neural crest state. Our data suggests that HDACs play an important role in retaining the cells that will become neural crest cells in a stem cell state through gastrulation and neurulation, even as other cells in the early embryo become lineage restricted.
I was intrigued by your finding that HDAC inhibited cells co-expressed several lineage markers at once. What do you think explains these cells’ confused identities?
AR That is a great question. It seems that HDAC activity must be critical for keeping the acetylation state of genes controlling a number of different lineage states low in pluripotent stem cells, so that these genes are not expressed before the cells have made a specific lineage decision. Inhibition of HDAC activity releases this control, and therefore causes low-level expression of markers of multiple lineages simultaneously. An apparent consequence of co-expressing genes that promote multiple different lineage states is an inability to commit to any one lineage. During normal development, signals instructive of a specific lineage state likely release HDAC control of gene expression essential to that lineage, while ensuring that other lineage control factors remain unexpressed.
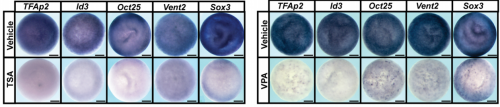
What do you think might be downstream of HDAC in the neural crest, and do you expect these targets to be the same as in the naïve blastula?
AR & CLB We know that global levels of H3K9 and H3K27 acetylation are similar in blastula and neural crest stem cells, and we think that the key targets of HDAC activity are likely to be similar in both of these cell types. We have used RNA-Seq to characterize the changes to the transcriptome that occur in response to HDAC inhibition, and that gives us important clues to what the key targets might be. We are currently examining the deposition of specific acetylation marks genome wide using ChIP-Seq to investigate this further.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
AR I was really excited when I found that increasing HDAC activity enhanced the ability of blastula cells to be reprogrammed to a neural crest state. I think it definitely was my eureka moment, because it gave us the fundamental insight that HDACs actively promote the retention of pluripotency that leads to formation of the neural crest.
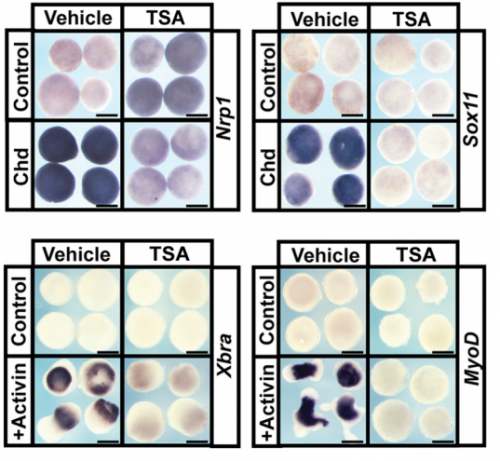
And what about the flipside: any moments of frustration or despair?
AR My most frustrating moments during this project were actually during the revisions for the paper. The reviewers asked us to confirm the phenotypes we observed following chemical inhibition of HDAC activity by using an independent means of down-regulating this activity. This was very challenging because HDAC1 is very highly expressed in the early embryo. However, with Carole’s guidance, I was able to target a morpholino that blocks HDAC1 translation to the neural crest, and show that this phenocopied the effects of the chemical inhibitors.
What next for you after this paper?
AR I am currently diving deeper into understanding the epigenetic regulation of the neural crest using ChIP-Seq and proteomics, and I hope to gain further insights into the role of specific epigenetic marks in stem cell maintenance.
And where will this work take the LaBonne lab?
CLB We are continuing to drill down on the epigenetic control of pluripotency in both naïve blastula cells and neural crest cells, and in developmental decision making more generally. Ultimately we want to understand these processes at single cell resolution using both quantitative imaging and single cell genomics. We are fortunate to be a part of a new NSF-Simons Center for Quantitative Biology focused on understanding emergent properties in developmental biology. Our project is focused on understanding dynamical transitions in cell states, and we have amazing math and modelling collaborators in the Center who are going to help us to take these studies to whole new levels of analysis.
Finally, let’s move outside the lab – what do you like to do in your spare time in Illinois?
AR In my spare time, I enjoy running on the beautiful trail along Lake Michigan and I am currently training to run the New York City marathon in November. I also love reading, particularly historical fiction, and trying out new restaurants around Chicago.
CL I am the mother of two and the Chair of my department, so spare time is a bit of a foreign concept for me! In the time I do have I love to cook, travel and take long walks along the lake with my two standard poodles.
Histone deacetylase activity has an essential role in establishing and maintaining the vertebrate neural crest
Anjali Rao, Carole LaBonne
Development 2018 145: dev163386 doi: 10.1242/dev.163386
This is #48 in our interview series. Browse the archive here.


 (1 votes)
(1 votes)
A nice one!
Keep it up!