In Development this week (Vol 137, Issue 20)
Posted by Seema Grewal, on 28 September 2010
A fateful look at early mouse lineage specification
The first cell lineages specified in the mouse embryo are the trophectoderm (TE), which generates the embryonic portion of the placenta, and the inner cell mass (ICM). The ICM subsequently forms the pluripotent epiblast (EPI, which produces the embryo) and the primitive endoderm (PrE, which generates other extraembryonic structures). Two papers shed new light on these crucially important early embryonic specification events.
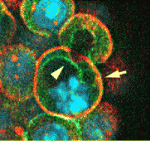 On p. 3383, Janet Rossant and colleagues investigate the role that the intercellular adhesion molecule E-cadherin plays in the divergence of the TE and ICM. By embryonic day 3.5, the TE has formed a polarised epithelial layer that encloses the apolar ICM. The researchers show that the normal epithelial morphology of the TE is disrupted in mouse embryos lacking both maternal and zygotic E-cadherin function but that individual cells in the blastocyst still initiate TE- and ICM-like fates. Interestingly, most of the cells express the TE marker Cdx2, which suggests that organised epithelium formation is not necessary for TE-specific gene expression. Furthermore, individual cells in these embryos still generate an apical membrane domainthat correlates with Cdx2 expression. Thus, the epithelial integrity mediated by E-cadherin is not required for Cdx2 expression but is essential for setting normal TE/ICM ratios in mouse embryos.
On p. 3383, Janet Rossant and colleagues investigate the role that the intercellular adhesion molecule E-cadherin plays in the divergence of the TE and ICM. By embryonic day 3.5, the TE has formed a polarised epithelial layer that encloses the apolar ICM. The researchers show that the normal epithelial morphology of the TE is disrupted in mouse embryos lacking both maternal and zygotic E-cadherin function but that individual cells in the blastocyst still initiate TE- and ICM-like fates. Interestingly, most of the cells express the TE marker Cdx2, which suggests that organised epithelium formation is not necessary for TE-specific gene expression. Furthermore, individual cells in these embryos still generate an apical membrane domainthat correlates with Cdx2 expression. Thus, the epithelial integrity mediated by E-cadherin is not required for Cdx2 expression but is essential for setting normal TE/ICM ratios in mouse embryos.
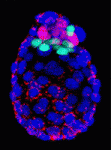 On p. 3361, Anna-Katerina Hadjantonakis and colleagues identify a role for platelet-derived growth factor (PDGF) signalling in PrE expansion in mouse embryos. The PDGF receptor-α (PDGFRα) is an early marker of the PrE lineage and of extra-embryonic endoderm (XEN) cells, which can be isolated from mouse blastocysts as derivatives of the PrE lineage. By combining live imaging and lineage analysis, the researchers show that Pdgfra expression coincides with that of GATA6, the earliest expressed transcription factor in the PrE lineage. GATA6 expression, they report, is required for Pdgfra transcriptional activation, and PDGF signalling is essential for the establishment and proliferation of XEN cells in culture. Moreover, implantation-delayed Pdgfra-null mutant blastocysts contain reduced PrE cell numbers and, surprisingly, increased EPI cell numbers, indicating that reciprocal signalling between PrE and EPI tissues might regulate compartment size within peri-implantation mammalian embryos.
On p. 3361, Anna-Katerina Hadjantonakis and colleagues identify a role for platelet-derived growth factor (PDGF) signalling in PrE expansion in mouse embryos. The PDGF receptor-α (PDGFRα) is an early marker of the PrE lineage and of extra-embryonic endoderm (XEN) cells, which can be isolated from mouse blastocysts as derivatives of the PrE lineage. By combining live imaging and lineage analysis, the researchers show that Pdgfra expression coincides with that of GATA6, the earliest expressed transcription factor in the PrE lineage. GATA6 expression, they report, is required for Pdgfra transcriptional activation, and PDGF signalling is essential for the establishment and proliferation of XEN cells in culture. Moreover, implantation-delayed Pdgfra-null mutant blastocysts contain reduced PrE cell numbers and, surprisingly, increased EPI cell numbers, indicating that reciprocal signalling between PrE and EPI tissues might regulate compartment size within peri-implantation mammalian embryos.
For more on early mouse lineage segregation, see also the review by Fredrik Lanner and Janet Rossant on p. 3351.
OMA-1/2: repressors of translation and transcription
 Primordial germ cell specification requires global transcriptional repression. In C. elegans, the zygote (P0) undergoes four successive asymmetric divisions to generate the germline precursors P1, P2, P3 and finally P4, the germline founder. OMA-1 and OMA-2 (OMA1/2), cytoplasmic proteins degraded after the first mitotic cycle, repress global transcription in P0 and P1 by sequestering TAF-4, an RNA polymerase II pre-initiation complex component, while the maternal protein PIE-1 represses transcript elongation in P2-P4. Now, Rueyling Lin and colleagues report that OMA proteins repress transcription in P2-P4 indirectly by maintaining PIE-1 expression (see p. 3373). OMA-1/2, they show, repress zif-1 mRNA translation in oocytes; zif-1 encodes the substrate-binding subunit of the E3 ligase that marks PIE-1 for degradation. MBK-2, a kinase that is activated after fertilisation, controls OMA1/2 function, report the researchers. Thus, they suggest, MBK-2 phosphorylation of OMA1/2 acts as a key developmental switch in the oocyte-to-embryo transition by converting OMA proteins from specific translational repressors in oocytes to global transcriptional repressors in embryos.
Primordial germ cell specification requires global transcriptional repression. In C. elegans, the zygote (P0) undergoes four successive asymmetric divisions to generate the germline precursors P1, P2, P3 and finally P4, the germline founder. OMA-1 and OMA-2 (OMA1/2), cytoplasmic proteins degraded after the first mitotic cycle, repress global transcription in P0 and P1 by sequestering TAF-4, an RNA polymerase II pre-initiation complex component, while the maternal protein PIE-1 represses transcript elongation in P2-P4. Now, Rueyling Lin and colleagues report that OMA proteins repress transcription in P2-P4 indirectly by maintaining PIE-1 expression (see p. 3373). OMA-1/2, they show, repress zif-1 mRNA translation in oocytes; zif-1 encodes the substrate-binding subunit of the E3 ligase that marks PIE-1 for degradation. MBK-2, a kinase that is activated after fertilisation, controls OMA1/2 function, report the researchers. Thus, they suggest, MBK-2 phosphorylation of OMA1/2 acts as a key developmental switch in the oocyte-to-embryo transition by converting OMA proteins from specific translational repressors in oocytes to global transcriptional repressors in embryos.
Shh: new TWISTs to limb patterning
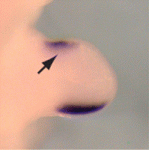 Sonic hedgehog (SHH) controls anterior-posterior (A-P) patterning in the mammalian limb. Its expression is normally restricted to the posterior limb bud but when expressed ectopically, it can change digit number and/or identity. Several transcriptional factors regulate Shh expression in the limb bud but how do they function together? On p. 3417, Xin Sun and co-workers report that interactions between two negative regulators of Shh expression (the ETS transcription factors ETV4/5 and the bHLH transcription factor TWIST1) and a positive regulator of Shh expression (the bHLH transcription factor HAND2) control A-P limb patterning in mice. By examining mutant limb buds, the researchers show that Twist1 is required to inhibit Shh expression in the anterior limb bud, and that it acts with the Etv genes to antagonise Hand2. Moreover, biochemical data indicate that the ETV proteins inhibit Shh expression by regulating the dimerisation of TWIST1/HAND2. Together, these findings highlight the importance of a precise balance between positive and negative regulators of Shh expression during limb A-P patterning.
Sonic hedgehog (SHH) controls anterior-posterior (A-P) patterning in the mammalian limb. Its expression is normally restricted to the posterior limb bud but when expressed ectopically, it can change digit number and/or identity. Several transcriptional factors regulate Shh expression in the limb bud but how do they function together? On p. 3417, Xin Sun and co-workers report that interactions between two negative regulators of Shh expression (the ETS transcription factors ETV4/5 and the bHLH transcription factor TWIST1) and a positive regulator of Shh expression (the bHLH transcription factor HAND2) control A-P limb patterning in mice. By examining mutant limb buds, the researchers show that Twist1 is required to inhibit Shh expression in the anterior limb bud, and that it acts with the Etv genes to antagonise Hand2. Moreover, biochemical data indicate that the ETV proteins inhibit Shh expression by regulating the dimerisation of TWIST1/HAND2. Together, these findings highlight the importance of a precise balance between positive and negative regulators of Shh expression during limb A-P patterning.
Muscling in on motoneuron specification
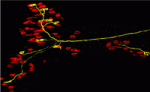 Mammalian skeletal muscles contain several types of muscle fibres, each characterised by its contraction speed and molecular properties. Individual motor axons innervate a few dozen muscle fibres, usually all of the same type. How this striking ‘motor unit homogeneity’ is established is incompletely understood but, on p. 3489, Joshua Sanes and colleagues reveal that, in mice, signals from the muscle fibres influence the molecular properties of motoneurons that innervate them. The lack of markers for motoneuron types has impeded the study of motor unit homogeneity. Here, however, the researchers show that the motoneurons that innervate slow muscle fibres selectively express the synaptic vesicle protein SV2A and carry it to their nerve terminals. Notably, overexpression of the transcriptional co-regulator PGC1α in muscle fibres, which converts them to a slow phenotype, increases the number of SV2Apositive motoneurons. The researchers propose, therefore, that retrograde signals from muscles integrate with previously described anterograde influences of the nerve on the muscle fibre to match the properties of these synaptic partners to each other.
Mammalian skeletal muscles contain several types of muscle fibres, each characterised by its contraction speed and molecular properties. Individual motor axons innervate a few dozen muscle fibres, usually all of the same type. How this striking ‘motor unit homogeneity’ is established is incompletely understood but, on p. 3489, Joshua Sanes and colleagues reveal that, in mice, signals from the muscle fibres influence the molecular properties of motoneurons that innervate them. The lack of markers for motoneuron types has impeded the study of motor unit homogeneity. Here, however, the researchers show that the motoneurons that innervate slow muscle fibres selectively express the synaptic vesicle protein SV2A and carry it to their nerve terminals. Notably, overexpression of the transcriptional co-regulator PGC1α in muscle fibres, which converts them to a slow phenotype, increases the number of SV2Apositive motoneurons. The researchers propose, therefore, that retrograde signals from muscles integrate with previously described anterograde influences of the nerve on the muscle fibre to match the properties of these synaptic partners to each other.
LIN-42-ing up development and stress
 Environmental stresses, such as nutrient fluctuations, can affect developmental progression in animals. C. elegans larvae, for example, normally develop into adults through four larval stages under the control of heterochronic (developmental timing) genes such as lin-42, a homologue of the circadian rhythm gene period. But, when times are hard, C. elegans forms long-lived dauer larvae, an alternative third larval stage. Now, Ann Rougvie and co-workers report that lin-42 functions in dauer entry (see p. 3501). Loss of lin-42, they report, makes animals hypersensitive to dauer formation under stressful conditions, whereas misexpression of lin-42 in pre-dauer stages inhibits dauer formation. Other experiments suggest that LIN-42 acts in opposition to the ligand-free form of the nuclear receptor DAF-12, which integrates external cues and developmental decisions. Together, these results suggest that LIN-42 and DAF-12 are intimate partners in the decision to become a dauer larva and raise the possibility that Period-like proteins play a conserved role in coordinating intrinsic timing mechanisms with environmental conditions.
Environmental stresses, such as nutrient fluctuations, can affect developmental progression in animals. C. elegans larvae, for example, normally develop into adults through four larval stages under the control of heterochronic (developmental timing) genes such as lin-42, a homologue of the circadian rhythm gene period. But, when times are hard, C. elegans forms long-lived dauer larvae, an alternative third larval stage. Now, Ann Rougvie and co-workers report that lin-42 functions in dauer entry (see p. 3501). Loss of lin-42, they report, makes animals hypersensitive to dauer formation under stressful conditions, whereas misexpression of lin-42 in pre-dauer stages inhibits dauer formation. Other experiments suggest that LIN-42 acts in opposition to the ligand-free form of the nuclear receptor DAF-12, which integrates external cues and developmental decisions. Together, these results suggest that LIN-42 and DAF-12 are intimate partners in the decision to become a dauer larva and raise the possibility that Period-like proteins play a conserved role in coordinating intrinsic timing mechanisms with environmental conditions.


 (No Ratings Yet)
(No Ratings Yet)