Where does blood come from in the first place and how is it made?
Posted by Rui Monteiro, on 14 September 2016
Commentary on
Transforming Growth Factor β Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells
in Developmental Cell, Volume 38, Issue 4, p358–370, 22 August 2016
Each of us has around 6 pints of blood. The blood contains a number of different types of cells, including oxygen-transporting red blood cells, disease-protecting white blood cells or wound-closing platelets. But have you ever wondered where they all come from?
Quite amazingly, all these very different blood cells originate from the same parental cell, called the haematopoietic stem cell (HSC for short). HSCs live inside our bone marrow and keep making new blood cells throughout life. That’s why you don’t have to worry if you cut yourself and lose some blood – your bone marrow will make new cells very quickly. In fact, a single haematopoietic stem cell has the potential to make all 6 pints of your blood!
As it turns out, the way we make the first HSCs is very similar to all other vertebrates studied so far (Ciau-Uitz et al, 2014): they come from endothelial cells, the cells lining the vessels of the circulatory system. But only a specialised type of endothelium gives rise to HSCs – the haemogenic endothelium, located in the main artery of the 6-week old human embryo. 100 years ago, Emmel had observed blood cells associated with arterial endothelium in pig embryos (Fig.1).
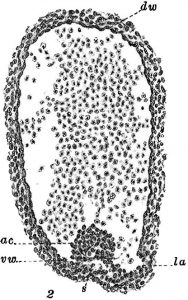
At that time, similar observations were made in a miriad of other vertebrate embryos, including the mongoose, the chick, the rabbit and the human (see Adamo and Garcia-Cardena, 2012 for the full references). This abundance of early observations led to the hypothesis that blood cells came from… blood vessels! The evidence to support this very simple hypothesis didn’t come until 2010, when a few research groups imaged the birth of an HSC in live zebrafish embryos (Bertrand et al., 2010; Kissa and Herbomel, 2010; Lam et al., 2010).
In the Patient lab, we use zebrafish to find out what makes these endothelial cells, already part of a differentiated tissue, become our all-important HSCs. In our recent Developmental Cell paper, we solved another piece of the puzzle: we showed that the cytokine Transforming Growth Factor β (TGFβ) is needed very early in the developing embryos to make the endothelium become haemogenic, so that it can make HSCs. Here is the story of how we got there.
Why TGFβ?
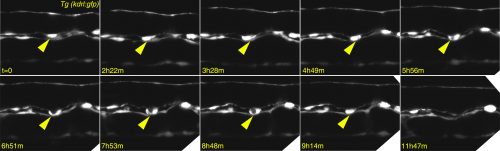
Any haematologist will tell you that if you give TGFβ to adult mice, their blood stem cells will stop proliferating and, if exposed for too long, they will die. So why would you need TGFβ to make the stem cells in the first place and why on Earth would you bother to even look at TGFβ? Well, the clue comes from epithelial cancers: an oncologist will tell you that TGFβ is a tumour suppressor that keeps cancer cells from proliferating and even from moving to other parts of the body…until it doesn’t! After a fatal tipping point, TGFβ becomes an oncogene and actually encourages the cancer cells to transform and go invade other tissues. This is what led us to look at TGFβ in the formation of HSCs – an endothelial cell leaving its place in a functional vessel and becoming an HSC (see Figure 2 for our own live imaging of the birth of an HSC) is remarkably similar to a metastasizing cancer cell!
This was a great opportunity to put together my interest in developmental biology and in stem cells, and hopefully contribute with new discoveries that may also be relevant to human biology. The first signs were encouraging: the TGFβ receptor tgfβR2 and its ligands tgfβ1a and tgfβ1b were present in the main embryonic artery at the right time; we also found tgfβ2 and tgfβ3 in the neighbouring notochord, further suggesting TGFβ signalling might play a role. Knocking down the receptor indeed led to the loss of haematopoietic cells, so we were in business! I convinced the British Heart Foundation that TGFβ was a good idea and got an Intermediate Fellowship to go ahead with this line of research, hosted in the Patient lab at the MRC Weatherall Institute of Molecular Medicine. You can find out more about other research ongoing at the MRC WIMM on the WIMM blog.
The first pieces of the puzzle
After we demonstrated that TGFβ was required to make the HSCs, we wanted to figure out how it related to other pathways known to play a role in the process. We turned to NanoString technology, a neat multiplex hybridization technology to look at what happened to gene expression downstream of TGFβ. Of the 100 plus genes we looked at, one turned out to be activated by TGFβ – the Notch ligand jag1a. This was a crucial finding, because jag1 had already been described as a target of TGFβ in metastasizing cancer cells (Zavadil et al., 2004) and so the link to Notch signalling was already established. We followed this lead and discovered that switching off jag1a also resulted in losing HSCs. Moreover, forced expression of jag1a rescued the loss of HSCs in TGFβ-deficient embryos, further supporting the TGFβ-Notch link.
Through a series of experiments that involved switching off other important cell signalling pathways and subsequent gene expression analysis, we managed to place the TGFβ pathway downstream of VEGF – a signal that is a well known player in the development of blood and blood vessels.
The final piece
At this point, we were excited that we had a nice VEGF-TGFβ-Notch story to tell, but we were not finished yet! We wanted to see which of the TGFβ ligands was doing the job. There are a number of different family members (TGFβ1, TGFβ2, TGFβ3), all of which trigger very similar events in their target cells. To our surprise, not only our prime suspects (based on expression analysis), TGFβ1a and b were required, but also TGFβ3 played a role. Even more surprisingly, TGFβ3 was more important at a later stage, when the HSCs actually leave the endothelium and become motile. Sticking to the similarities with cancer, this would be when the cancer cells metastasize. In short, while TGFβ1 and TGFβ3 were required to make the HSCs, they came from different sources and were required at different times. This finding really puts emphasis on how important it is to consider the timing of events when studying embryonic development.
Is this the end of the story?
No!… This is really just the beginning. What we discovered is that there is a ‘window of opportunity’ where TGFβ is required, but we and others have shown that if you have too much TGFβ you will also struggle to make HSCs (Nimmo et al., 2013; Vargel et al., 2016; Yang et al., 2016). Think of this like you’re cooking a meal: when you add salt, too much or too little of it will ruin your dish, so it’s important to get it right. How can we reconcile both observations? Also, how does TGFβ3 ‘take over’ the role of the main TGFβ ligand and how is it regulated? This is one of the most exciting times in the life of a researcher – when you get an answer that comes with… many more questions!
How can understanding the origins of our blood be useful in the long term? If we discover enough pieces of the puzzle, we may be able to write down a trusted ‘recipe’ to prepare the haematopoietic stem cells in a laboratory dish, step by step. Such optimised, lab-grown HSCs would have a great potential to help people suffering from various blood disorders, including leukaemias. Let’s hope we’ll be able to start ‘cooking’ soon!
Contributors: Rui Monteiro and Tomasz Dobrzycki
References
Adamo, L., and Garcia-Cardena, G. (2012). The vascular origin of hematopoietic cells. Dev Biol 362, 1-10.
Bertrand, J.Y., Chi, N.C., Santoso, B., Teng, S., Stainier, D.Y., and Traver, D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108-111.
Kissa, K., and Herbomel, P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112-115.
Lam, E.Y., Hall, C.J., Crosier, P.S., Crosier, K.E., and Flores, M.V. (2010). Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116, 909-914.
Nimmo, R., Ciau-Uitz, A., Ruiz-Herguido, C., Soneji, S., Bigas, A., Patient, R., and Enver, T. (2013). MiR-142-3p controls the specification of definitive hemangioblasts during ontogeny. Dev Cell 26, 237-249.
Vargel, Ö., Zhang, Y., Kosim, K., Ganter, K., Foehr, S., Mardenborough, Y., Shvartsman, M., Enright, A.J., Krijgsveld, J., and Lancrin, C. (2016). Activation of the TGFβ pathway impairs endothelial to haematopoietic transition. Scientific reports 6, 21518.
Yang, Q., Liu, X., Zhou, T., Cook, J., Nguyen, K., and Bai, X. (2016). RNA polymerase II pausing differentially regulates signaling pathway genes to control hematopoietic stem cell emergence in zebrafish. Blood.
Zavadil, J., Cermak, L., Soto-Nieves, N., and Bottinger, E.P. (2004). Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23, 1155-1165.


 (2 votes)
(2 votes)
I am going to try this again. It will be a new Dr. The first 2 times I tried to take blood, to get better. I was given the blood within 30 minutes. I was so ill. I was ill for the entire process. My 2 times were given too soon, I felt I was dying the whole month. Really what can I do.