The people behind the papers – Elena Popa and Abigail Tucker
Posted by the Node Interviews, on 28 February 2019
This interview, the 58th in our series, was recently published in Development
While many vertebrates have multiple sets of teeth over their lifetime, some, like humans, have just a single set of replacement teeth (diphydonty), while others, like mice, manage with a single set (monophydonty). This diversity raises both evolutionary questions – how did different tooth replacement strategies evolve? – and developmental ones – what mechanisms prevent replacement teeth in animals that have lost them? A new paper in Development tackles these questions with a molecular analysis of mouse tooth development. We caught up with first author Elena Popa and her supervisor Abigail Tucker, Professor of Development and Evolution at King’s College London, to find out more about the work.

Abigail, can you give us your scientific biography and the questions your lab is trying to answer?
AT My lab is interested in how bodies are formed during development, both from a clinical perspective of understanding birth defects, but also from the point of view of understanding how evolution has shaped our bodies. I started out investigating tadpole tail development for my PhD with Jonathan Slack and then swapped ends and moved to the head for my first postdoc with Paul Sharpe. Here, I investigated how the face and dentition are patterned. This is where I first encountered tooth development, and although I have moved on to study the cranial neural crest, the jaw, cranial glands and ear, I have always kept some experiments going to understand more about the tooth. Teeth are often the only thing left preserved in the fossil record, so they have a central importance to our understanding of evolution. There are still lots of unanswered questions, such as what regulates tooth number, tooth size and tooth shape? It’s a great model for understanding epithelial-mesenchymal interactions, as both tissues are integral to the formation of the final tooth but take it in turns to play the leading role.
Elena, how did you come to join the Tucker lab and what drives your research?
EP Developmental biology was by far my favourite subject during my undergraduate years at Royal Holloway. I didn’t know it was going to be tooth development in particular back then, but as soon as I read about Professor Abigail Tucker’s research I was completely captivated. Her lab provided the opportunity to perform a comparative study of molecular interactions and developmental processes that allow or disrupt tooth replacement in a wide variety of vertebrates. Why can’t we have more than two sets of teeth but snakes can? This was essentially the question that drove my research in the Tucker lab.
What initially led you to try and ‘reawaken’ tooth replacement in the mouse?
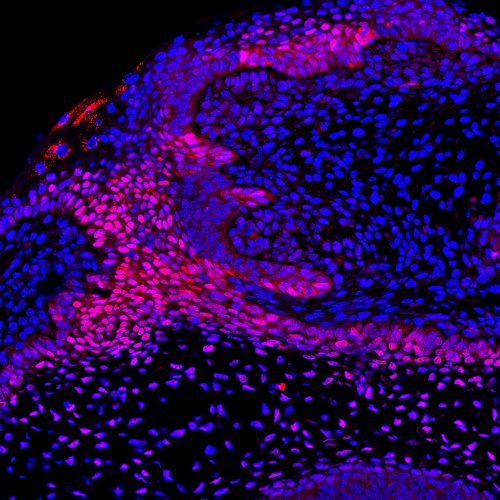
EP Why the potential for tooth replacement varies so much across vertebrates is an intriguing question. Having performed an in-depth study of the development of the dental lamina in many different animal models, we suspected that this structure retains the capacity to give rise to a subsequent generation of teeth, even in the molar region of the mouse (the mouse only has one generation of teeth and molars, in general, do not replace). The dental lamina next to the first molar can be seen protruding at E16.5 but disappears soon after birth, and has been termed the rudimentary successional dental lamina (RSDL). We compared the RSDL with the successional lamina of the minipig (which gives rise to a second generation tooth), and found that both expressed the epithelial stem cell marker Sox2; however, Wnt activity was only present in the minipig lamina. Knowing that stimulating Wnt signalling by means of different transgenic lines leads to the formation of supernumerary teeth, we based our experimental design on these comparisons, aiming to recapitulate tooth replacement in the mouse.
Can you give us the key results of the paper in a paragraph?
EP & AT Our results show that, although the mouse normally does not form a second replacement set of teeth, it still has the potential to do so if given the right signals. Stimulation of Wnt signalling in the rudimentary replacement lamina in transgenic mice or isolation of the lamina in culture both led to the formation of a new tooth. We started by showing that the RSDL exhibits molecular similarities to the competent dental lamina in a diphyodont mammal and retains odontogenic capacity, which we were able to reawaken by selectively inducing Sox2+ cells to activate canonical Wnt/β-catenin signalling. We were able to confirm the dental identity of the structures that arose from the RSDL in the mutant mice by performing in situ hybridization for genes known to be expressed during normal tooth development. The mutant RSDL was also highly proliferative and gave rise to multiple ectopic teeth, many of which were complex in shape and mineralised after transplantation in kidney capsule. We also uncovered an inhibitory relationship between Wnt signalling and Sox2, where ectopic stimulation of Wnt signalling leads to downregulation of Sox2 expression.
When you remove the first-generation tooth, this frees the RSDL to form a tooth bud. How is this potential inhibited in the context of normal development?
EP & AT Tooth number has previously been shown to be controlled by a balance between activators and inhibitors, creating an inhibitory cascade. For example, in many mammals three molars form at the back of the mouth by serial addition from a single molar placode. If the primordium for the subsequent molars is separated from the murine first molar in culture, the second molar initiates development faster and grows to a larger size than if left intact. The first molar therefore appears to be controlling the development of the next tooth in the series. In the shrew, the first generation of teeth initiate but then regress and are replaced by the permanent set of teeth. Here, again, it has been suggested that early formation of the permanent teeth might inhibit the development of the first set: timing and spatial arrangement of tooth germs is therefore clearly important in the control of final tooth number.
In our paper, we show that the RSDL has the potential to form a tooth and speculate that the adjacent molar sends a Wnt-inhibitory signal to the surrounding dental tissue. This then prevents Wnt activity in the RSDL, and leads to its regression. This is relevant to human tooth replacement, as structures similar to the RSDL have been identified next to the permanent teeth during development. In normal development of our dentition, therefore, the permanent tooth may inhibit the generation of a third set of teeth.
Wnt signalling leads to a reduction in Sox2 in the dental epithelium but not associated epithelia – what makes this relationship context dependent?
AT The context-dependent nature of the relationship between Wnts and Sox2 was very striking. This fits, however, with the literature, which has shown similar context-dependent interactions. For example, in the airway submucosa, Sox2 has both an inductive and a repressive effect on Wnt signalling that is dependent on the presence of other factors, whereas in the lung Wnts inhibit Sox2 but only at early stages of development. In the tooth, the repression of Sox2 by Wnts might be dependent on other factors with dynamic temporal and spatial expression patterns. It will be very intriguing to work out what these factors might be.
Do you know of any evolutionary scenarios where monophyodonty transitioned to diphyodonty, and if so does this involve a similar revitalisation to that you have discovered in the mouse?
EP & AT Throughout evolution, the general trend is one where animals reduce the number of tooth generations in favour of more-complex tooth shapes and better occlusion. As in the mouse, there is often evidence of a rudimentary structure, which points to this reduction in number. For example, we have shown that in the diphyodont fruit bat, the canine shows evidence of a third generation as it displayed a vestigial structure homologous to the mouse RSDL next to the second-generation tooth. In nature, there are rare cases proposed where teeth have been lost and then reappear, e.g. in the frog Gastrotheca guentheri, where teeth are found on the lower jaw but are absent in all other frogs. This would suggest that rudimentary tooth structures can be reawakened not just in the lab.
Did the research include any particular result or eureka moment that has stuck with you?
AT For me the eureka moment was when we generated a tooth germ from the RSDL by simply cutting off the main tooth. Really it’s a simple experiment but has a key message, which is that the reason a mouse doesn’t have a second set of teeth is that the first generation of teeth inhibits this from happening. This has important consequences, as it means that if this inhibition could be lifted an extra set of teeth might be possible.
And what about the flipside: any moments of frustration or despair?
EP For my PhD I worked with a lot of non-model organisms (bats, geckos, guinea pigs, opossum) in addition to the minipig and mouse shown here. These samples were always much more difficult to obtain and every time you wanted to look at gene expression it meant cloning, so things took much longer than expected. In addition, the anti-Sox2 antibody has been a particularly tricky to work with across species. Considering that it is at the core of my research, it became frustrating when it simply refused to work. After what felt like hundreds of failed attempts, finally being able to see Sox2 staining under the microscope felt like a huge relief!
After what felt like hundreds of failed attempts, finally being able to see Sox2 staining under the microscope felt like a huge relief!
So what next for you after this paper?
EP I have loved my time in science and particularly in the Tucker lab, where I had the opportunity to learn a great deal and diversify my wet lab skillset beyond what I could have ever hoped for. I’ve now shifted my focus to science media production, observing and documenting scientific discoveries from behind a camera lens.
Where will this work take the Tucker lab?
AT For me the next question is what signals from the first tooth stop the second generation forming? Our results predict that such signals prevent canonical Wnt signalling from being activated in the RSDL. Expression patterns predict possible roles for dickkopf 2 and dickkopf 3 that could be tested in culture or in vivo. Another avenue is the vestibular lamina. This is a really understudied structure but appears to have the potential to form teeth when stimulated by Wnt signalling. I am really interested in the relationship between the dental lamina and vestibular lamina, as these two epithelial structures are united during early development, forming from the same placode. What signals determine whether you become a dental lamina and form teeth, or a vestibular lamina and form the cheek ridge, is next on my list.
Finally, let’s move outside the lab – what do you like to do in your spare time in London?
EB I think the UK is an incredibly beautiful country, so I tend to spend most of my free time travelling and exploring its fantastic landscapes. My favourite places to go are Cornwall and the Lake District. I also have a characterful little Whippet puppy, who takes up a lot of my time at the moment!
AT I commute into London from Kent where I live with my husband, children, cat, bearded dragon and five snakes. I love London, having grown up there, but have become a convert to the countryside. I love cooking, eating and travel, and am writing this from the Atacama desert in Chile.


 (3 votes)
(3 votes)