The people behind the papers – Zhi Ye and David Kimelman
Posted by the Node Interviews, on 21 February 2021
This interview, the 91st in our series, was published in Development last year.
The anterior to posterior extension of the vertebrate body axis relies on a population of bipotent neuromesodermal progenitors in the tailbud. A new paper in Development uncovers a crucial and unexpected new role for Hox13 genes in sustaining these progenitors to promote axis extension in zebrafish. To hear more about the story, we caught up with the paper’s two authors: postdoctoral researcher Zhi Ye and his supervisor David Kimelman, Professor of Biochemistry and Adjunct Professor of Biology at the University of Washington, Seattle.

David, can you give us your scientific biography and the main questions your lab is trying to answer?
DK: As a graduate student at Harvard, I worked on adenovirus, but I became fascinated by developmental biology and had the great fortune to work as a postdoc with Marc Kirschner on early Xenopus development at UC San Francisco, where we did some of the founding studies on mesoderm-inducing factors. When I started my own lab in Seattle, I continued to study Xenopus, and eventually became intrigued with the possibilities in zebrafish, which is the current focus of my lab.
The main problem we are interested in has fascinated developmental biologists for almost a century, which is how the posterior body forms from the mass of cells called the tailbud. This problem has been reinvigorated recently with a finding that we and others made: that the mesoderm and neural tissue of the posterior body arise from a very interesting neuromesodermal progenitor population. There is now a terrific collection of scientists working in this area and I am very happy to be part of this international effort.
And Zhi, how did you come to join David’s lab, and what drives your research today?
ZY: Three years ago, after I obtained my PhD from a genetics research group at Auburn University that studies farmed fish, I decided to obtain training in a developmental model system to better equip myself for an academic career. I am specifically interested in the field of developmental biology as it aims to uncover the mysterious, yet gorgeous, mechanisms that allow a single cell to develop into such a diverse variety of different organisms. In addition, I wanted to learn cutting-edge technologies that will be useful in advancing my scientific career.
When David responded to my application with a long email giving me some details about his lab, I could feel his enthusiasm and passion about science through these words. We had an interview where I was introduced to the Hox project, which really impressed me, and fortunately he agreed to train me. I feel so lucky that I made the right decision to join David’s lab and had the chance to work with the other friendly and outstanding developmental biologists at the UW. The three years of training in David’s lab has been such a great experience, not only because I have learned a lot about how to do research, but also, which I think is even more important, how to be a responsible scientist and mentor.
How has your research been affected by the COVID-19 pandemic?
ZY: Like many other people, I was not able to do bench work during the 6 weeks of full lockdown at the UW, and things were also slowed down as some reagents we needed from Germany for a key experiment were delayed for months. It turned out to be a good time for me to do some intensive reading and to learn computational skills for the bioinformatics analysis of the sequencing data that is the basis for my next paper.
DK: I spend a lot of time driving back and forth between my house, where I now have my office set up, and the lab, where my experiments are. Research feels very disjointed because of this.
What led you to initially work with zebrafish Hox13 genes?
DK: I have spent a lot of time trying to think about how the tailbud cells continually and progressively release cells into the mesoderm as the body axis extends. I was particularly intrigued by the overexpression studies in amniotes that proposed that the Hox13 genes act to terminate this process. My original plan was to see if we could just extend the amniote studies by doing overexpression in zebrafish, and then isolating the tailbud cells and performing RNA-seq to analyse the genes controlled by the Hox13 proteins, as the previous studies had only studied a few candidate downstream genes.
Can you give us the key results of the paper in a paragraph?
ZY & DK: Whereas the previous studies had argued that the Hox13 genes act to terminate body axis extension in vertebrates, our results show that they act together with the transcription factor Brachyury (now called Tbxta in many systems) to promote the formation of mesoderm from the neuromesodermal progenitors, thereby allowing the posterior body to form. This is a very different way of looking at the role of the Hox13 genes, which we believe will be applicable to all vertebrate embryos.
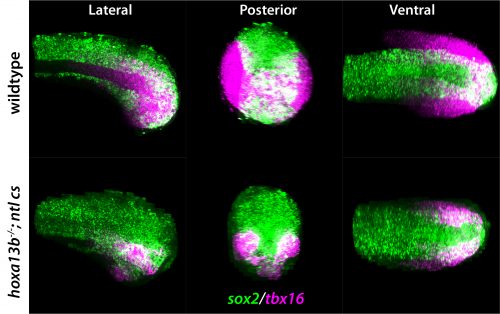
I understand the path from making the Hox13 mutants to finding a phenotype relied on some fortunate coincidences?
DK: As stated above, the original idea was to overexpress the Hox13 genes and then analyse gene expression in the tailbud with RNA-seq, as no one had comprehensively studied the downstream genes. We spent a lot of time doing this, but honestly it was a mess because, although lots of genes went up and lots of genes went down, we couldn’t make any sense of what we were seeing, and I felt terrible that I had dragged Zhi into this hellhole of a project. Fortunately, CRISPR had become relatively easy in zebrafish, and I was making a lot of mutants in genes of interest, and so I made a mutant in one of the two most abundant Hox13 genes: hoxa13b. However, to my disappointment it had no effect. I then targeted the other abundant Hox13 gene, hoxd13a, to make a hoxa13b;hoxd13a double mutant, and then I did see a small percentage of embryos with some phenotype, but it was really a major bummer after all that work and many months of raising and screening fish that the effect was so marginal.
Very fortunately I asked Zhi to give me his thoughts on the double mutant embryo phenotype, and he asked me to put the embryos at 21°C after fertilization rather than the normal 29°C, as this would allow him to watch the body form during regular work hours (at 29°C much of the body extension occurs during the middle of the night with zebrafish). I did as he requested and I was really surprised when he told me that he was seeing dramatic effects on axis formation in many of the embryos! At first I thought he had to be mistaken, but he was indeed correct. What we now know is that we were just very lucky that I had made the Hox13 mutations in the background of a homozygous cold-sensitive mutation in tbxta, which is a naturally occurring mutation that is present among the lab’s ‘wild-type’ fish. This tbxta mutant normally has no effect, such that fish with just the tbxta cold-sensitive mutation are completely normal when grown at 21°C, but when the two Hox13 genes are mutated in this background there is a very strong synergistic effect. Had I not by chance made Hox13 CRISPR mutants in a homozygous tbxta cold-sensitive background, and had Zhi not asked me to raise the embryos at 21°C, we would not have uncovered this whole story. Serendipity has often been a major factor in my scientific career, and this work completely exemplifies this point.
Serendipity has often been a major factor in my scientific career, and this work completely exemplifies this point
When doing the research, did you have any particular result or eureka moment that has stuck with you?
ZY: When I saw the larger neural tube and smaller presomitic mesoderm in the hoxa13;hoxd13 double CRISPR mutants, I was really surprised and excited! I had been working on studying RNA-seq data from the Hox13 overexpression lines for more than a year but I couldn’t make any sense of how the transcriptional changes caused by overexpressing Hox13 produced such a profound truncation of the embryos. The fate change phenotype shown in the double mutants provided us with a whole new way of thinking about the role of the Hox13 genes in early embryos.
And what about the flipside: any moments of frustration or despair?
ZY: This was a very tough project for me as we went through so many ups and downs. For example, when I told David my exciting findings with the CRISPR mutants, David warned me that as I had kept the embryos at a cooler temperature to slow them down for analysis, it might be an artefact due to the fact that some of our ‘wild-type’ fish have the cold-sensitive tbxta mutation, which would have been a huge disappointment. But it was a very lucky break as it is only when Tbxta function is reduced that the role of eliminating hox13 genes is revealed. The interaction of the Hox13 proteins and Tbxta helped us develop a new understanding of the role of the Hox13 proteins. The beauty of science, and also life, is that all these upsetting moments can also lead to great happiness. I am so grateful to David for his continuous support and encouragement during the hard times in this project.
What next for you after this paper?
ZY: We are working on finishing up a second exciting story about the Hox13 genes in which we identified the direct targets of Hox13 in vivo, and these results strongly back up the findings in our first paper. We plan to submit this work soon. After leaving David’s lab, I am going back to China and I will spend time studying interesting targets of Hox13 that we were not able to cover in our second paper. I am currently seeking a faculty position that will allow me to continue this work; combining my postdoctoral research with my graduate studies, I plan eventually to use a developmental biology perspective to improve aquaculture production.
Where will this story take the Kimelman lab?
DK: Zhi has adapted a very cool new method called CUT&RUN that has allowed him to identify genes that the Hox13 factors bind in vivo. One of the major problems with the studies of all Hox proteins, particularly in vertebrates, has been determining real in vivo targets; Zhi’s work has identified many interesting target genes. While Zhi will study some in his own future lab, there are plenty of targets for both of us to analyse.
Finally, let’s move outside the lab – what do you like to do in your spare time in Seattle?
ZY: Hiking is absolutely my first choice. Seattle is such a wonderful place to live with a variety of natural landscapes and hiking trails available within a 2-hour driving distance. Cooking is another way of relaxing; it’s like doing experiments but you can get your results (dishes) and publication (photos on social media) much quicker!
DK: For the past 10 years I have been working on Sundays with a terrific organization called Rebuilding Together Seattle, for which I go to houses throughout the Seattle metropolitan area and do free home repairs for people on a low income. It is a bit like science in that I am constantly trying to solve problems, but also very gratifying in that I can directly improve people’s lives.


 (No Ratings Yet)
(No Ratings Yet)