AdamTS matrix metalloproteases mediate basement membrane heterogeneity required for organ elongation
Posted by Uwe Töpfer, on 4 July 2024
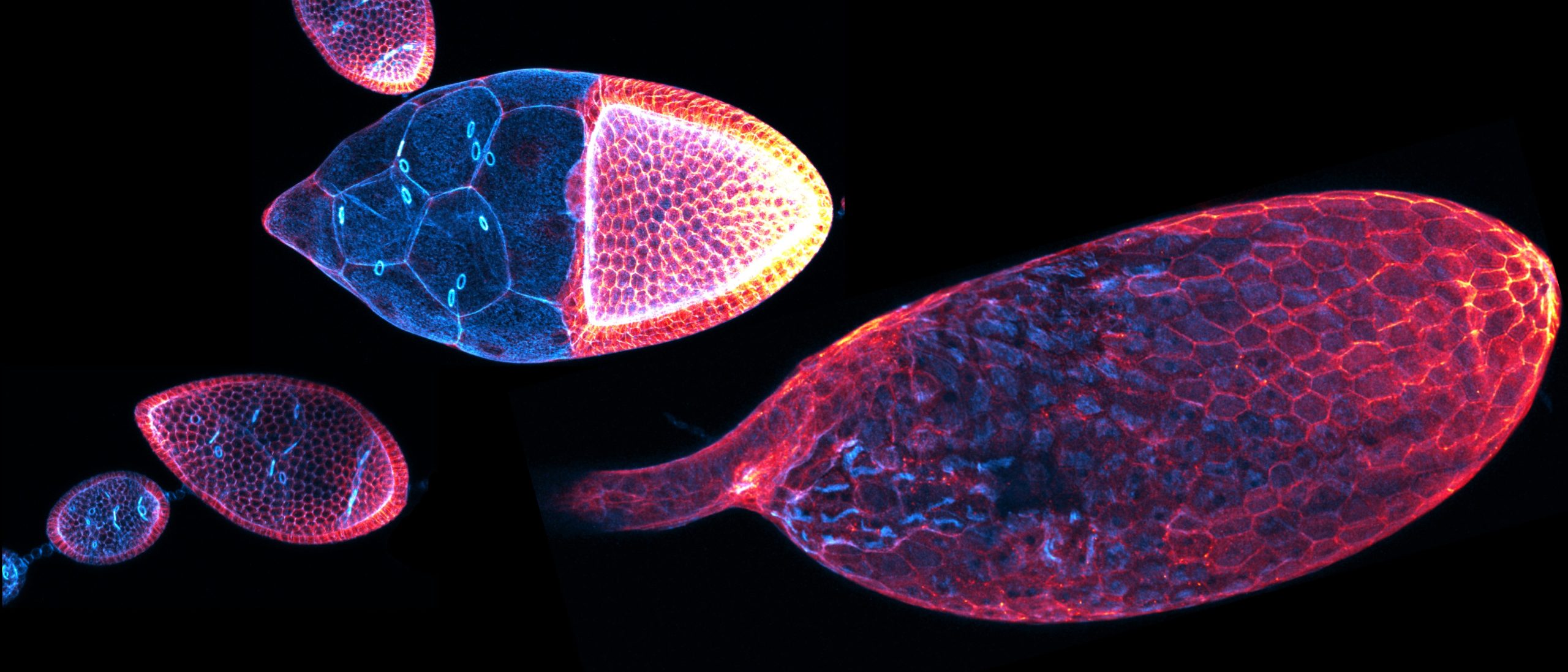
Basement membranes (BMs) are thin, specialized extracellular matrices that surround most tissues and organs (Jayadev and Sherwood, 2017). These meshworks serve as scaffolds for cell adhesion, influencing cell signaling, cell migration, proliferation, and differentiation (Sherwood, 2021; Yurchenco, 2011). Moreover, dysregulation of BM remodeling lead to disturbed tissue and organ development or disease (Sekiguchi, R and Yamada, K. M., 2018). Recent publications indicate that establishment of BM heterogeneity might be important for tissue and organ sculpting (Agarwal et al., 2022; Harmansa et al., 2023; Harunaga et al., 2014; Kyprianou et al., 2020; Serna-Morales et al., 2023; Uwe Töpfer et al., 2022). However, how this heterogeneity is induced and how this leads to organ sculpting is largely unknown.
Agarwal, P., Shemesh, T. and Zaidel-Bar, R. (2022). Directed cell invasion and asymmetric adhesion drive tissue elongation and turning in C. elegans gonad morphogenesis. Developmental Cell 57, 2111-2126.e6.
In our recent publication (Töpfer et al., 2024), we identified two AdamTS matrix proteases required for the proper elongated shape of the egg chamber. Knockdown of stall or AdamTS-A results in rounder eggs from early elongation phase on. While the phenotypes look very similar, the molecular mechanisms by which they act are different.
Using CRISPR/Cas, we tagged both proteins with sfGFP and found a dynamic expression, resulting in higher protein enrichment in the terminal regions (at the most anterior and posterior regions). We were able to detect Stall in early stalk cell precursors and stalk cells as well as a strong expression later in the polar cells with a gradual expression at the terminal regions of stage 8 egg chambers. AdamTS-A was uniformly expressed in all somatic precursors of follicle cells, but becomes more strongly enriched at the terminal regions in stage 8 egg chambers, too.
Next, we used fly lines with GFP-tagged ECM components to study the proteases’ role in ECM remodeling. We found that Stall is required to establish basement membrane heterogeneity by locally limiting Collagen IV protein density. In contrast to a lower fluorescence signal in control egg chambers in the posterior region, stall knockdown led to a nearly uniform protein level of Collagen IV along the anterior -posterior axis.
Using high-resolution microscopy, we studied the pattern of fiber-like structures embedded in the BM. We found that the knockdown of AdamTS-A results in a disturbed BM micropattern. BM fiber-like structures were shorter and smaller in AdamTS-A knockdown egg chambers. Maturation (length and proper orientation) of BM fiber-like structures has been associated with egg chamber rotation. Accordingly, we also found that AdamTS-A is required for proper egg chamber rotation, hence knockdown of AdamTS-A results in a premature stop.
We performed Atomic force microscopy to measure the stiffness of the BM. In both knockdown conditions, BM stiffness was globally increased, what goes along with increased apical pSRC level. Finally, we found slower E-Cad recovery in a FRAP experiment and a disturbed cell aspect ratio in the central regions. This data indicates that basement membrane remodeling by AdamTS-A and Stall influences gradual BM remodeling which induces BM stiffness and cell shape globally, which is required for organ shape.
References
Harmansa, S., Erlich, A., Eloy, C., Zurlo, G. and Lecuit, T. (2023). Growth anisotropy of the extracellular matrix shapes a developing organ. Nat Commun 14, 1220.
Harunaga, J. S., Doyle, A. D. and Yamada, K. M. (2014). Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Developmental Biology 394, 197–205.
Jayadev, R. and Sherwood, D. R. (2017). Basement membranes. Current Biology 27, R207–R211.
Kyprianou, C., Christodoulou, N., Hamilton, R. S., Nahaboo, W., Boomgaard, D. S., Amadei, G., Migeotte, I. and Zernicka-Goetz, M. (2020). Basement membrane remodelling regulates mouse embryogenesis. Nature 582, 253–258.
Sekiguchi, R and Yamada, K. M. (2018). Basement Membranes in Development and Disease. Current Topics in Developmental Biology 130, 143–191.
Serna-Morales, E., Sánchez-Sánchez, B. J., Marcotti, S., Nichols, A., Bhargava, A., Dragu, A., Hirvonen, L. M., Díaz-de-la-Loza, M.-C., Mink, M., Cox, S., et al. (2023). Extracellular matrix assembly stress initiates Drosophila central nervous system morphogenesis. Developmental Cell 58, 825-835.e6.
Sherwood, D. R. (2021). Basement membrane remodeling guides cell migration and cell morphogenesis during development. Current Opinion in Cell Biology 72, 19–27.
Töpfer, U., Guerra Santillán, K. Y., Fischer‐Friedrich, E. and Dahmann, C. (2022). Distinct contributions of ECM proteins to basement membrane mechanical properties in Drosophila. Development 149 (10): dev200456.
Töpfer, U., Ryu, J., Guerra Santillán, K. Y., Schulze, J., Fischer-Friedrich, E., Tanentzapf, G. and Dahmann, C. (2024). AdamTS proteases control basement membrane heterogeneity and organ shape in Drosophila. Cell Reports 43, 114399.
Yurchenco, P. D. (2011). Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harbor Perspectives in Biology 3, a004911–a004911.


 (No Ratings Yet)
(No Ratings Yet)