BSDB Gurdon Summer Studentship Report (13)
Posted by BSDB, on 30 October 2017
When I tell my friends that I spent my summer looking down a microscope at worms they often give a snigger. My enthusiasm on the subject quickly earned me the affectionate (I hope) nickname, ‘Wormboy’. Yet the Wormboys and girls of Richard Poole’s lab instilled in me a great thirst for scientific study over the 8 weeks I spent with them, which I will certainly carry forward in my career. The lab studies the nematode worm C. elegans, and my job was to help characterise a glia-to-neuron transition they discovered in the male tail that occurs during sexual maturation. During my time, I learned how to handle worms, keep them healthy, perform crosses, use a fluorescent microscope and present my data to an audience. All of this as part of my first lab experience was not only invaluable, but very enjoyable, and I came away wanting more.
Worm death was a regular part of lab life. Saving them from starvation and disease was a constant uphill battle, one that at first I found insurmountable. Along with those natural causes of death, they also had to contend me, often crushing them clumsily under my pick and purging potentially contaminative stragglers on said pick in the Bunsen. Happily for the worms, such fates became less and less frequent, and I was eventually able to sustain healthy populations from which I selected young males to dunk in the intensely toxic chemical sodium azide, all the better to view them under fluorescent microscopy. A necessary sacrifice.
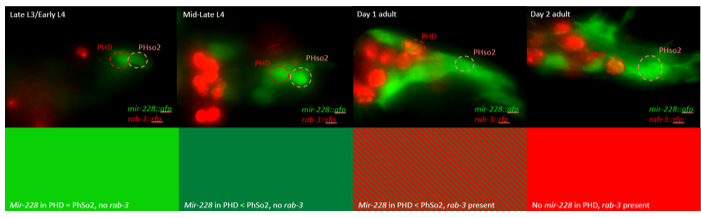
This microscopy work was the first step in characterising the glia-to-neuron transition of phasmid socket 1 (PhSo1) into the neuron PHD, which occurs in the male tail during sexual maturation. The lab had previously identified a similar transition of the amphid socket to MCM neuron in the head of the male (Sammut et al., 2015), though the PHD transition differs in how the neuron is formed. Where the amphid socket divides asymmetrically to generate the MCM, the PHD derives from PHso1 by direct trans-differentiation, without a division, while its sister- PHso2- remains glial. This new neuron’s activity was found to be linked to initiation of a novel behaviour employed by males during mating thought to improve their chance of spicule insertion. To track the changing identity of PHso1, I compared its expression of glial and neuronal markers linked to GFP and RFP respectively with that of PHso2. The two glial markers I used were grl-2 (socket specific protein) and mir-228 (pan-glial microRNA), and the neuronal marker a nuclear synaptic fusion protein, rab-3.
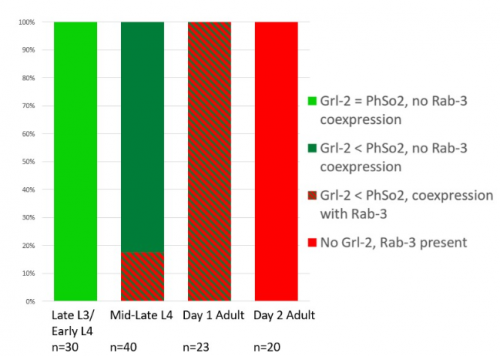
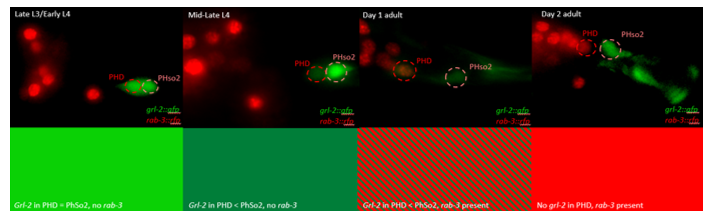
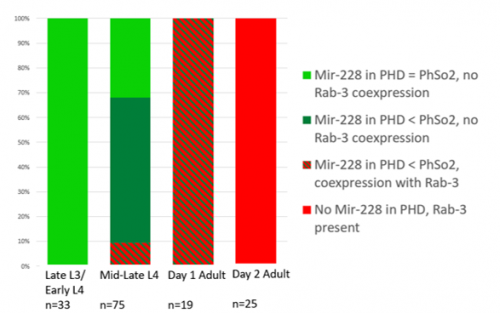
The glia-to-neuron transition appears to start in males of larval instar 4 (L4), while the gonad is taking shape. Through picking many, many worms, I showed a clear gradient of change of marker expression in PhSo1 compared with PhSo2:
- In late L3/early L4 GFP brightness is equal in the two sockets
- By mid/late L4, GFP is dimmer in PHso1
- The PHso1 of day 1 adults is beginning to express neuronal marker, displaying coexpression of GFP and RFP
- By day 2, all GFP has dissipated from the newly formed PHD
- Both glial markers displayed the same trend (fi g.1 and 2)
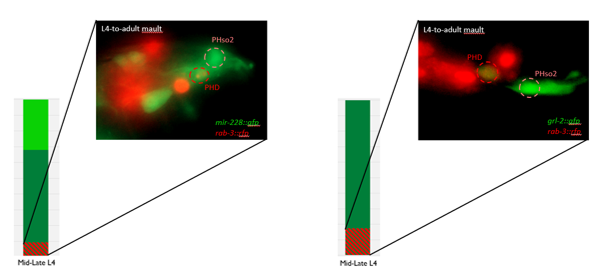
- Some animals classified mid/late L4 displayed coexpression of markers, but these were all on the cusp of adulthood, undergoing moult at the at the time of imaging (fig. 3)
Of course, fluorescent proteins do not perfectly match levels of the markers they represent, rather they give a general overview of the transformation. At the very least, this result shows PhSo1 might partially dedifferentiate before it eventually acquires neuronal characteristics when the worm becomes an adult. Understanding trans-differentiation events such as this is key to understanding how nature itself reassigns cell fate. And if you’re trying to do something yourself in cell biology, it often pays to learn how nature beat you to it. To determine the mechanics of this trans-differentiation would require a more quantitative technique: single molecule fluorescent in situ hybridisation (smFISH).
SmFISH employs fluorescently tagged RNA oligomers, antisense to an mRNA (or miRNA) of interest. Combined, these oligos fluoresce strongly enough that individual RNAs can be resolved and counted within PHso1. Tracking change in RNA quantity rather than GFP fluorescence would tell you whether or not glial expression stops before neuronal expression begins. Does the cell become completely naive? Perhaps only partially? Or maybe there’s no dedifferentiation at all, and the cell passes through a totally novel identity- part glia, part neuron. Though I didn’t have time to perform these experiments, I did create the necessary strains. By crossing a markerless strain with my GFP/RFP animals, and then selecting against RFP over a couple of generations I rendered worms expressing only one of the two GFP markers so that the RFP reporter used in smFISH would be visible.
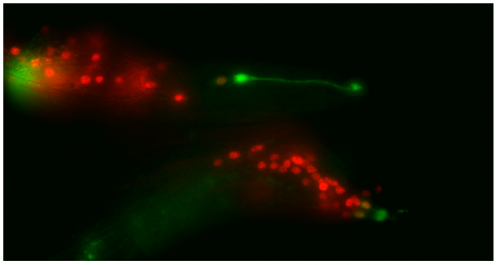
Though the work I was doing represented a project in its early stages and was at times very laborious, I quickly got an appetite for it. There was a thrill to it, knowing that no one had ever done what I was doing. I felt that buzz again when I arranged the prettiest pictures I could muster into figures to show to the lab, and then again when they ceremoniously scoured my findings with the same level of scrutiny I had seen them exact on each other. Alongside my data, I showed off a particularly pretty picture of both the worm’s glia-to-neuron transitions occurring at once in the same animal, which I think will remain my crowning achievement in science for a long time (Richard even kindly offered to steal it for his presentations). The BSDB’s grant has allowed me a taste of what a career in academia can offer. Straightforward experiments fraught with unforeseen difficulties. Working on weekends when, infuriatingly, age-specific experiments simply weren’t possible on Friday. Enough money to live off (just). But above all, the enormous reward in discovering something new. And getting to work with some really, really great people. Thanks guys.
References
Sammut, M., Cook, S., Nguyen, K., Felton, T., Hall, D., Emmons, S., Poole, R. and Barrios, A. (2015). Glia-derived neurons are required for sex-specific learning in C. elegans. Nature, 526(7573), pp.385-390.


 (5 votes)
(5 votes)