Christmas Confetti lights to understand the embryonic origins of blood
Posted by MIGUEL GANUZA FERNANDEZ, on 1 November 2017
![]()
My PhD focused in deciphering molecular mechanisms implicated in cell cycle regulation in embryonic and adult tissues. As many others in this field I became fascinated by the experiments by Yamanaka and colleagues and this prompted me to transition into the stem cell world. For this reason I joined Shannon McKinney-Freeman’s laboratory in 2012. Here, I became interested in understanding how Hematopoietic Stem Cells (HSCs) arise during embryonic development.
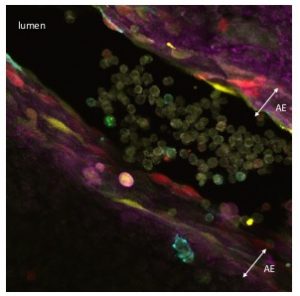
HSCs are mesodermal in origin and emerge from a subset of endothelial cells (known as hemogenic endothelium) mainly in the major arteries of the embryo. The hemogenic endothelium undergoes and endothelial-to-hematopoietic transition during midgestation. This process can be visualized as clusters of cells budding from the arterial endothelium into the lumen of these embryonic arteries.
In order to better understand how these clusters are formed I thought it would be interesting to analyze the expression profiles of cell cycle regulators within these cells in murine embryos. I was discussing some of these results with a brilliant former postdoc in the lab, Per Holmfeldt. He mentioned the existence of a genetic tool (the Confetti allele) that had been used to study the behaviour of other types of stem cells for example in the intestine (Snippert et al., 2010). I thought it was a great idea that could bring some light into the formation of these clusters.
The Confetti allele is a conditional multicolored reporter composed of four fluorescent proteins (GFP, CFP, YFP and RFP) flanked by different lox P sites in particular orientations. In the unrecombined orientation the cassette does not lead to the expression of any fluorescent protein. Upon Cre expression the Confetti allele recombines and the cells get randomly and stably label with one of the four colors. Importantly all the progeny of a particular cell will maintain the expression of the same color.
As I started working with this genetic tool (Figure 1, video 1), I realized that it could be used to answer more sophisticated questions. In particular, I thought it could be employed to understand long-standing issues like the clonal origin of the adult blood system at different embryonic stages.
In mammals, all previous studies have estimated that blood emerges from just a handful of blood progenitors during embryonic development. All these studies were based on the transplantation of embryonic tissues, that had to be previously dissected, dissociated, sometimes cultured ex vivo and finally transplanted. I thought all these stresses could have an impact on the final readout. Here a non-invasive approach should better reveal the actual clonal embryonic origin of the adult blood.
The challenge part here was that the Confetti allele only allows to label the blood with up to four colors. So it did not seem enough to study larger complexities. But here I had an idea.
Let’s say that we have a bag full of marbles of four different colors. These marbles can be green, yellow, blue or red. If we ask two persons to grab one marble. It is very likely that the marble that each of them have grabbed is different. In contrast, if each of these persons pick 100 marbles and then they compare the distribution of the colors they got it is more likely that it will be more similar. The reason is that the more marbles they get the distribution of the colors will be a better representation of what it is in the bag.
In this line, since the Confetti cassette labels the cells randomly with one of four colors (yellow, green, blue or red), this same mathematical concept could be applied for our purposes. The more blood precursors (marbles picked) then the distribution of the confetti colors among the blood of adult mice (persons grabbing marbles) will be more similar. If the number of blood precursors was small, then the distribution of the Confetti colors among different mice would be very different.
Here, you can probably imagine that the idea was now to use the mouse-to-mouse variance in the distribution of the Confetti colors in the adult blood as a predictor to estimate the number of blood precursors. Hence the required experiment was to plate known numbers of Confetti cells and then for each cell dose calculate the well-to-well variance in the distribution of the Confetti colors.
I have loved Mathematics all my life and this has been critical for me to develop this idea.
Still, I did not have the right expertise to derive a formula that could correlate these two variables. In order to crystallize this concept, we brought into the project a fantastic statistician, David Finkelstein, how was able to create a formula where we can use the mouse-to-mouse variance in the distribution of Confetti colors in the adult blood to infer the number of blood precursors.
After a number of experiments to validate this formula we were ready to apply this equation to our major question. We generated cohorts of mice where blood precursors were “Confetti-labeled” at particular developmental stages. Finally, we were able to estimate that lifelong hematopoiesis is established by hundreds of embryonic precursors at different stages during mouse development (Ganuza et al., 2017).
For me, this project has been extremely instructive further than from just the exciting data we found. I think it is a good example that illustrates how important is to interact with your colleagues in the lab to get new ideas that could lead to a completely new project. It definitively shows how important is to merge different scientific disciplines to address previous or new questions employing a fresh approach. I deeply believed that Mathematics need to be more heavily used in Biology.
References
- Ganuza M, Hall T, Finkelstein D, Chabot A, Kang G, McKinney-Freeman S. Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny Nat Cell Biol. 2017 Oct;19(10):1153-1163.
- Snippert HJ et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010 Oct 1;143(1):134-44.


 (No Ratings Yet)
(No Ratings Yet)