Continuous and Extended Ex Utero Embryogenesis in Mammals
Posted by Alejandro Aguilera Castrejon, on 6 May 2021
By Alejandro Aguilera Castrejon and Jacob H. Hanna
Understanding the developmental processes leading to the formation of tissues and organs represents one of the most fundamental questions in developmental and stem cell biology. In mammals, most of this process takes place after the embryo implants inside the maternal uterus. After implantation, mammalian embryos initiate the process of gastrulation, in which stem cells differentiate into the three germ-layers, and subsequently commence organ formation, transiting from a symmetrical ball of stem cells into an advanced embryo with defined head, tail, organs and limbs. The intrauterine confinement of developing embryos has limited the study of post-implantation embryogenesis, due to the inability to observe and manipulate living embryos at these stages. Further, the very small size of the early post-implanted embryo makes it extremely difficult to observe inside the uterus using techniques applied for monitoring development of older fetuses. Thus, for over a century, scientists have attempted to devise ex utero culture systems capable of supporting mammalian post-implantation embryo growth, and had very limited success.
The motivation for our group to initiate this project was brought by the lack of methods for continuous and robust culture of early post-implanted embryos until advanced organogenesis, while at the same time, we have been able to routinely culture mouse embryos in vitro through all stages of pre-implantation development for decades. It is also not feasible to transfer explanted post-implantation embryos back to the uterus as commonly done for pre-implantation embryos. More specifically, we embarked on this project following our success to get low contribution of human derived iPSC cells into cross-species chimeric mouse embryos for the first time (Gafni et al., 2013), as we wanted to follow the integration of human iPSC derived cells in post-implantation mouse embryos, and also try to improve it (Bayerl et al., 2020). Further, devising continuous in vitro culture systems for mammalian embryos during the period from pre-gastrulation until advanced organ formation would facilitate direct experimental investigation of key processes during post-implantation embryogenesis.
To the best of our knowledge, the earliest attempts to culture post-implanted rodent embryos started in the thirties by using rat plasma combined with embryo extract (Nicholas and Rudnick, 1934). Several research groups improved this technique over the years, by modifying different culturing parameters such as supplementing the embryo environment with different types of media and rotating the cultured embryos (New and Coppola, 1970; New, Coppola and Terry, 1973; P. P. Tam and Snow, 1980; Sadler and New, 1981; Rivera-Pérez, Jones and Tam, 2010; Piliszek, Kwon and Hadjantonakis, 2011). However, normal embryonic development in previous studies was inefficient, and limited to only brief periods of time after the post-gastrulation embryos were dissected out of the uterus (24-48 hours) (Sadler, 1979). Such short-term culture techniques do not allow to study embryogenesis comprehensively, regardless the age of the embryo isolated from the uterus, because most of the organs develop and mature over several days.
As an attempt to overcome these limitations, my supervisor Jacob Hanna thought about starting a project to revisit and re-evaluate the previously established short-term culture protocols and try to understand why they failed to obtain continuous embryogenesis ex utero. We reasoned that sensitive regulation of O2 and CO2 gas concentration is likely to be a critical parameter for embryogenesis. We also thought that controlling and increasing gas pressure could improve culture outcome and efficiency by increasing oxygen diffusion into the embryonic bloodstream, as routinely applied by lung ventilation machines used in hospitals (that also control gas pressure to enhance oxygen diffusion). However, there was no available system to control gas concentration and pressure precisely, so we engineered an electronic devise specially fitted for this purpose, which could be connected to the roller culture incubator (Fig. 1), and hired an external engineer to assemble it (Fig. 2a).
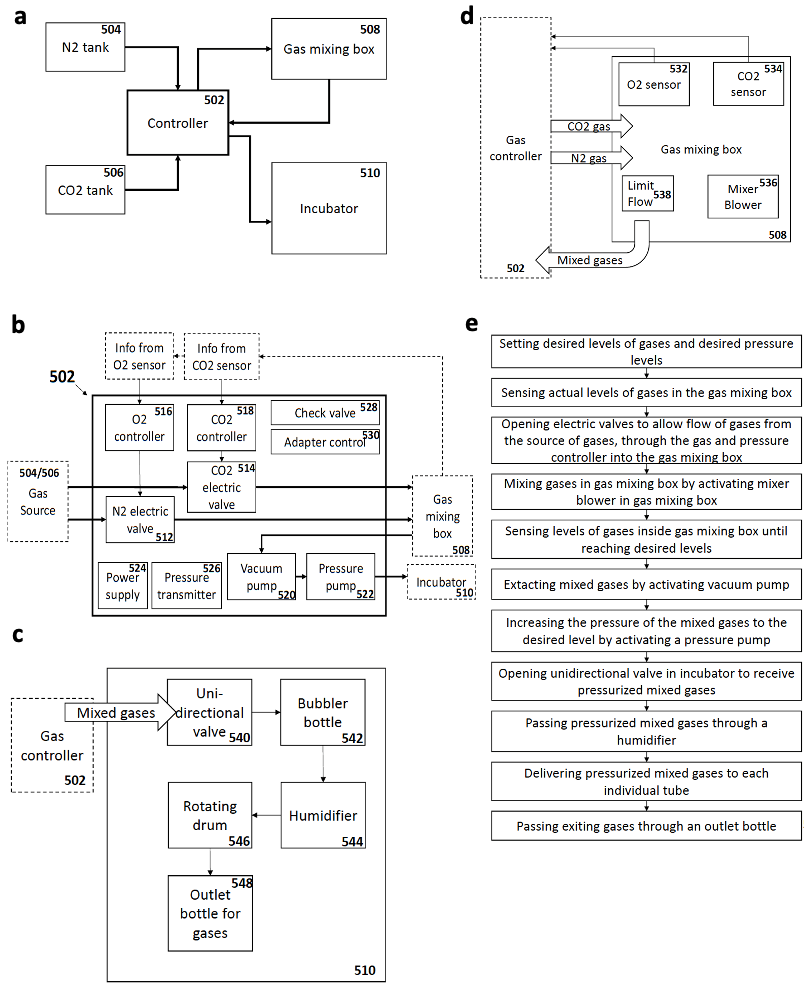
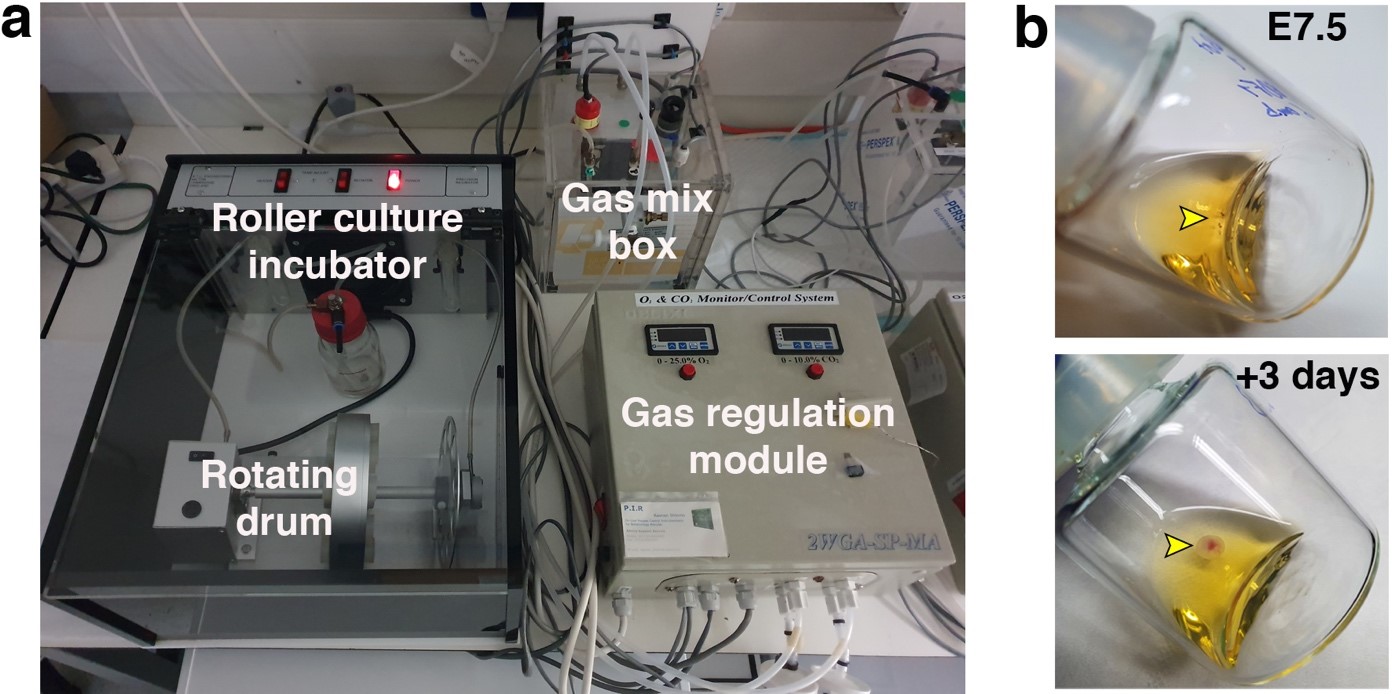
Once the gas regulation module was designed, and after I joined the lab as a master student, we started testing for different combinations media, oxygen concentrations, and gas pressures that allowed embryo growth with the highest survival rate and for the longest period of time. We tested different combinations of sera from different species, synthetic sera, nutrients and other supplements which have been used for culture of stem cells and embryos. By these means, we established a platform that supports growth of late gastrulating embryos at E7.5 until the hindlimb formation stage E11 by culturing the embryos in rotating bottles at hyperbaric pressure (Fig. 2b).
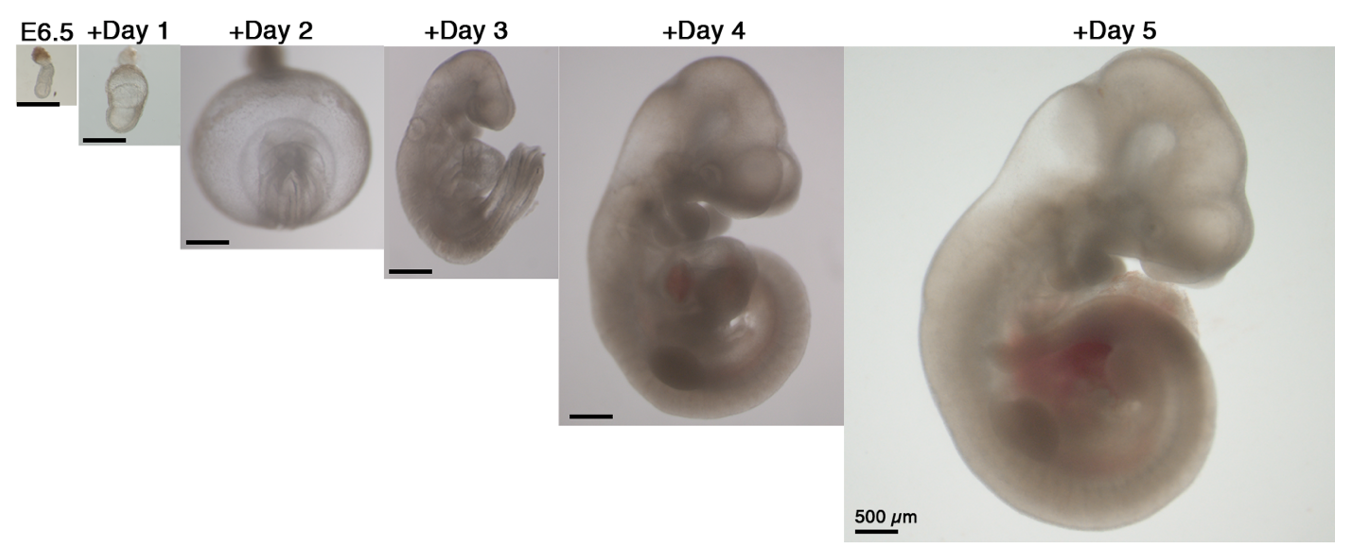
Our next step was to expand the ex utero culture platform one more day, aiming for starting with early gastrulating embryos at E6.5 until advanced organogenesis. However, no matter which conditions we tested, E6.5 embryos were not able to grow beyond two days using rotating bottle settings, possibly because E6.5 seemed very fragile when placed in rotating bottle conditions. At this stage we turned to devise cultures in static plates for early-gastrulation stage embryos, as we realized that there was not a well-established protocol for static culture of embryos at E6.5, since the culture conditions used per lab were variable, together with a disparity on efficiency and quality of embryo survival (P. P. L. Tam and Snow, 1980). We then decided to seek for conditions allowing robust development of embryos in static cultures from E6.5 until E8.5, since static culture does not support development beyond the early somite stage at E8.5. After testing diverse oxygen concentrations, gas pressures, types of sera, extracellular matrices and supplements we generated a protocol that allowed proper development of most explanted embryos. Remarkably, transfer of embryos cultured using our established static protocol to the roller culture allowed continuous growth from E6.5 to E11 (Fig. 3). Later, we realized that this static protocol is also suitable for growing embryos dissected at the earliest day of post-implantation development E5.5, and combined with the rotating bottles culture we were able to reach up to 6 days of mouse development ex utero.
Notwithstanding, a limitation of the protocol was the dependence on freshly isolated human umbilical cord blood serum, which availability can be limiting. Thereby, we intended to find another type of sera which could replace it. We tested first using commercial human blood sera, but the results were not encouraging, possibly because often commercial serum productions is not done rapidly after blood collection, which leads to increased hemolysis by-products that can be toxic to embryos. Thus, we turned to isolating serum from fresh human adult blood in-house, which is relatively easier to obtain. Indeed, we were very happy to find that freshly isolated adult human serum was able to support mouse embryo growth, which makes the protocol much more available to the community.
A fundamental step was to perform a thorough examination of the embryos in order to verify that those developing ex utero mimic their counterparts growing inside the uterus. By means of morphological, histological, immunofluorescence, and single cell transcriptomic analyses, we validated that the embryosdeveloping ex utero are comparable to embryos growing inside the maternal uterus at the level of tissue architecture and cell composition. In particular, for the single cell RNA-seq analysis we chose to examine embryos cultured in the extended static and roller culture protocol, since embryos cultured from E7.5 in rotating bottles are cultured for a shorter time than those starting at E6.5. It was very important for us to conduct single cell RNA-seq based comparisons, since the latter can be viewed as a very stringent and unbiased way of comparing embryos and their organs.
Furthermore, one of the key advantages of our ex utero culture is the amenability of the embryos to physical, chemical and genetic manipulations, that can be followed for over up to six days of development. For this purpose, we looked at all those experimental techniques that have been available widely for pre-implantation embryos, and that we have always fantasized to apply for studying post-implantation development, for instance, gene targeting, high resolution imaging, or generation of chimeric embryos by cell transplantation. First, we wanted to demonstrate the ability to introduce genetic perturbations in the developing embryos, for which we employed two strategies: on the one hand we used whole-embryo electroporation, which is usually transient and localized to a specific region; on the other hand, we carried out lentiviral transduction to introduce DNA in a stable and widespread manner all over the embryonic and extraembryonic tissues. For live cell imaging, we aimed to show the ability to image in high resolution using confocal microscopy. We chose to image two of the most intricate processes of embryonic development: gastrulation and neural tube closure. The first process is perfectly suitable for imaging in static culture, and for the second one we wanted to show the possibility to image embryos that were initially grown using the roller culture system and then moved to static conditions for imaging under the microscope while they continue developing. Finally, we have always been fascinated by the ability of cells to integrate into developing embryos to generate chimeric animals; procedure frequently done in our lab by grafting mouse or human pluripotent stem cells into mouse blastocysts, which can further develop by transfer to a surrogate mother. Nevertheless, such an assay was not available to evaluate the integration of cells into post-implanted mammalian embryos. In this regard, by analyzing the integration of mouse and human cells into gastrulating embryos, we demonstrated that our platform offers a novel opportunity to assay long-term integration of cells into mouse embryos at post-implantation stages. Overall, we proved that we can perform a variety of embryo manipulations without affecting embryo development or culture efficiency.
In summary, by establishing platforms for continuously growing pre-gastrulation mouse embryos outside of the uterus from the embryonic day 5 to 11, our recently published study (Aguilera-Castrejon et al., 2021) provides a proof-of-concept for the ability to continuously capture mammalian gastrulation and advanced organogenesis in an artificial environment, and underscores the self-organizing ability of the embryo. This culture system may help uncover mechanisms of cell fate specification and organ formation in a mammalian model, as it alleviates the uterine barrier to allow dynamic experimentation in living mouse embryos. We hope that our ex utero culture will be adopted by many labs around the world, and that culturing mouse embryos in a tube for extended periods of time will become a commonly used technique as culturing cells or embryos from non-mammalian species. Another interesting possibility for this system would be to use it in combination with synthetic stem cell-derived embryo models (Harrison et al., 2017), for which this platform could provide an ultimate test to evaluate their resemblance to natural embryos.
Leaving aside the ethical implications that the ex utero culture may have for human embryos, by creating these platforms for culturing mouse embryos we intend to investigate the intricate processes shaping mammalian organs during embryogenesis, which in the future, may have a broad range of implications for human health, from understanding congenital disorders and cancer, to stem cell biology and tissue engineering.
Aguilera-Castrejon, A., Oldak, B., Shani, T. et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature (2021). https://doi.org/10.1038/s41586-021-03416-3
References
There are dozens more ‘behind the paper’ stories featuring the highs and lows and serendipities of research on our dedicated page. Does your paper have a story behind it? We’d love to hear it.


 (5 votes)
(5 votes)