Deforming Nuclei: a way to move through the crowd!
Posted by Mariana Maia-Gil, on 3 February 2025
In their recent paper, Maia-Gil and colleagues explored whether and how nuclear properties can influence nuclear positioning in vivo. Their work revealed that in the densely packed retinal zebrafish neuroepithelium, nuclear deformability facilitates apical nuclear migration (Maia-Gil et al. 2024). Here, they share the science and the adventures that led to the development of this project.
What was already known?
The Norden Lab has been focused on understanding the cell and tissue biology behind zebrafish retinal development for 1.5 decades by now. The retina develops from a pseudostratified neuroepithelium, composed of a single, densely packed layer of highly elongated cells. Among other topics, the lab has been investigating a hallmark of such pseudostratified neuroepithelia which is apical nuclear migration before mitosis. This phenomenon is characterised by a fast and directed movement of nuclei toward the apical surface of the tissue. At the start of this project, we already knew:
- Why nuclei migrate apically: their apical positioning before cell division ensures tissue integrity (Strzyz et al. 2015).
- When this migration occurs: during the G2 phase of the cell cycle (Leung et al. 2011).
- How nuclei move: using an actin-dependent mechanism (Norden et al. 2009 and Yanakieva et al. 2019).
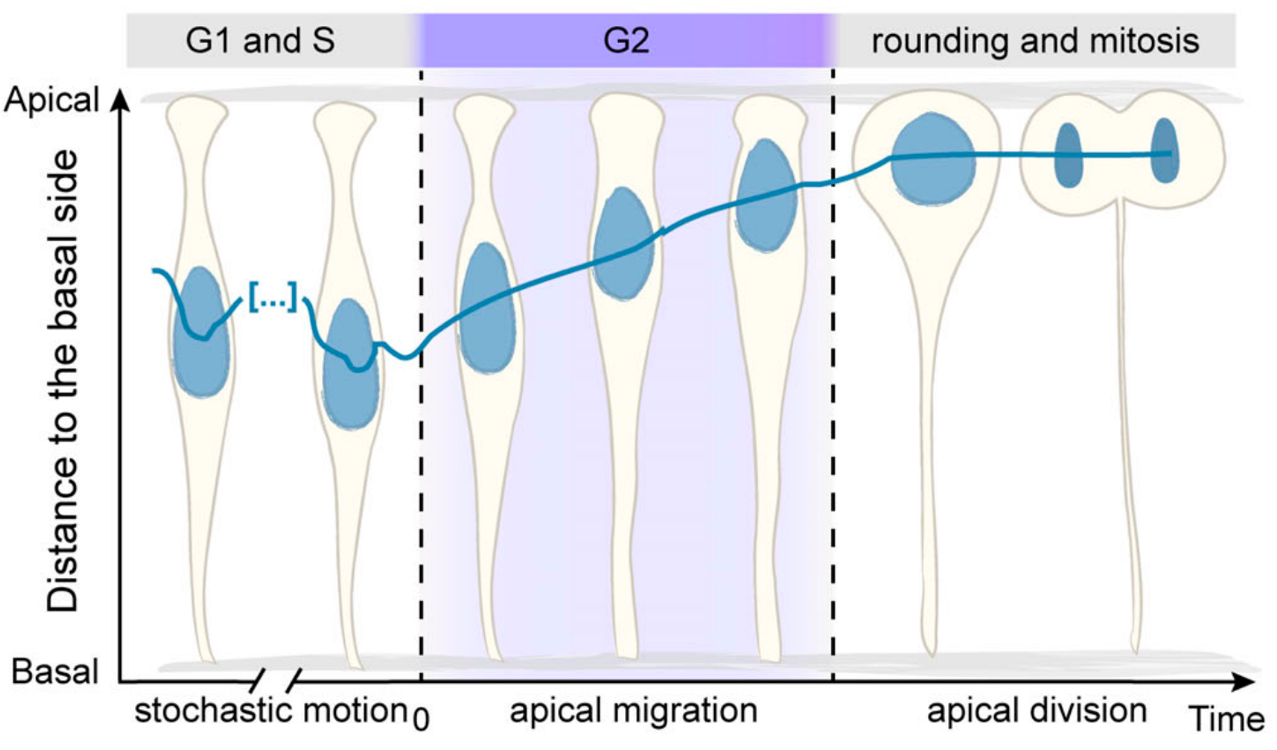
The big question: how to move through the crowd … and to get to the bar?
One evening, while the lab was out for a social gathering, we found ourselves facing a packed bar, and I was struggling to get my favourite drink – Porto Tónico. Determined to reach the bartender, we started brainstorming different ways to navigate through the crowd quickly and efficiently. In the middle of many creative (and impractical) ideas, someone jokingly suggested: “What if we could deform and squeeze through the crowd – just like nuclei do during apical migration?”
This offhand idea raised a novel question: Does nuclear deformability facilitate apical nuclear migration in the crowded retinal neuroepithelium?
Our approach: stiffening nuclei and tracking their movement during organ development
We already knew that zebrafish retinal nuclei are highly deformable and express low levels of Lamin A/C (Yanakieva et al. 2019), a nuclear envelope protein with expression level that correlates with nuclear stiffness. To test our hypothesis that nuclear deformability helps apical migration, we increased nuclear stiffness by using a previously generated transgenic zebrafish line in which all nuclei overexpress Lamin A (Amini et al. 2022). With crucial support from our collaborators, we used atomic force microscopy (with Elias Barriga and Jaime Espina) and developed a mechanical model that represents confined nuclei as compressible droplets (with Anna Erzberger and Roman Belousov) to confirm that Lamin A overexpression indeed led to stiffer nuclei in the retinal neuroepithelium.
The run to the apical side
With this knowledge and our established tools, we used light-sheet microscopy to image zebrafish retinas and quantify apical nuclear migration in vivo. We aimed to answer several key questions:
- Do stiffer, Lamin A overexpressing nuclei reach the apical side? Yes, but in contrast to control nuclei, stiffer nuclei take twice as long to cover equivalent distances. This delay occurs regardless of whether the surrounding nuclei are stiff or normal, suggesting that nuclear deformability facilitates migration in a cell-autonomous manner.
- Does the deformability of surrounding nuclei affect apical migration? Yes! Control nuclei surrounded by stiffer nuclei also take longer and show less directed movement. This indicates that the mechanical properties of the environment influence nuclear migration.
- Does increased nuclear stiffness impair apical migration in a less crowded epithelium? Here, the effect was less pronounced. Lamin A overexpression in the zebrafish hindbrain, a less crowded neuroepithelium, had only minor effects on apical nuclear migration. This suggests that the impact of nuclear stiffness depends on tissue packing.
- Does nuclear stiffness affect other processes requiring cellular deformation? Yes! Control cells took longer to round up before mitosis when in a stiffer environment, showing that nuclear properties can influence mitotic entry in a non-cell-autonomous manner.
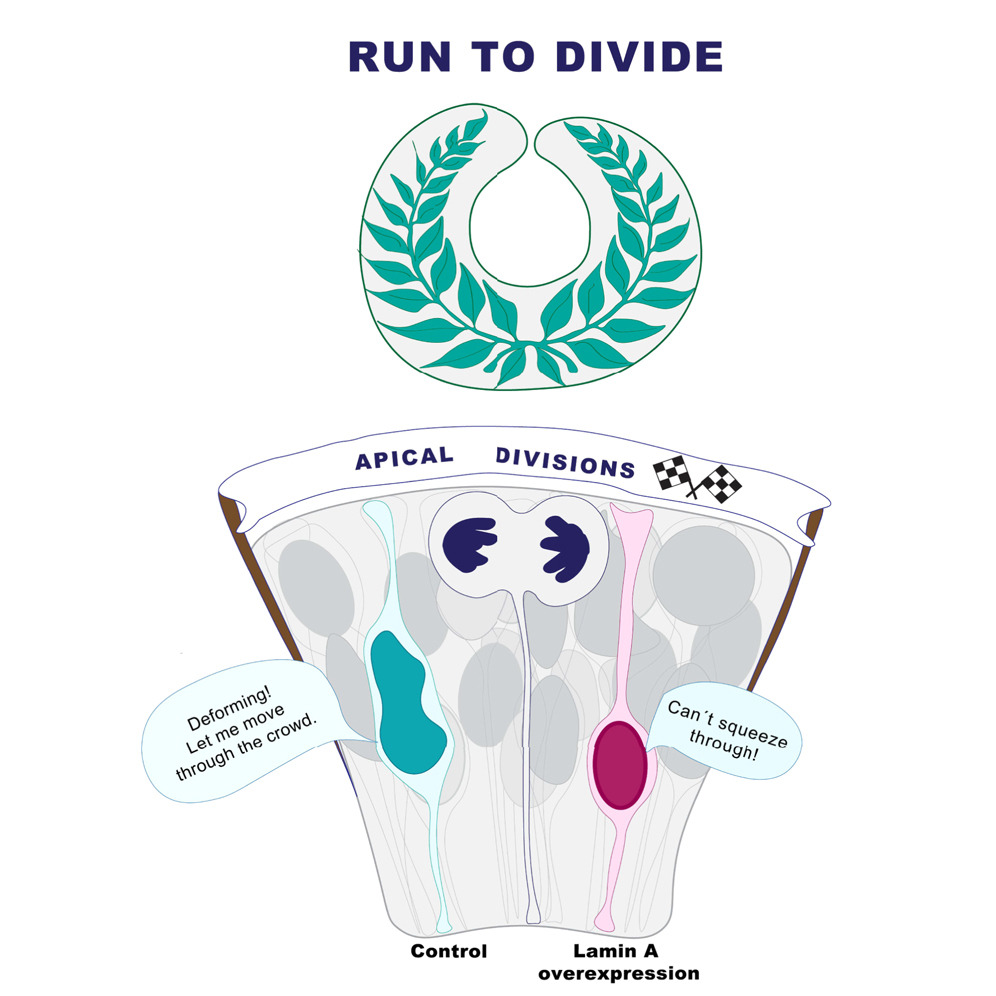
Together, these findings demonstrate that nuclear properties influence nuclear positioning and mitotic entry in a tissue-dependent manner during neuroepithelial development.
A personal developmental project…during revisions
My belly was growing, fatigue was setting in, and the time was ticking. The revision email arrived with a challenging to-do list. Priorities had to be redefined and our strategy adjusted. We needed more hands on the bench and had to dig deep into previous data. What could have been an erratic roller coaster turned into a successful and rewarding scientific adventure. I am grateful to have been surrounded by outstanding scientists who also advocate for woman in science. Their encouragement was invaluable – not only for the success of my PhD project but also for the development of the two most beautiful retinas I have ever seen.
References
1. Maia-Gil, M., Gorjão, M., Belousov, R., Espina, J.A., Coelho, J., Gouhier, J., Ramos, A.P., Barriga, E.H., Erzberger, A., and Norden, C. (2024). Nuclear deformability facilitates apical nuclear migration in the developing zebrafish retina. Current Biology 34, 5429-5443.e8. https://doi.org/10.1016/j.cub.2024.10.015.
2. Strzyz, P.J., Lee, H.O., Sidhaye, J., Weber, I.P., Leung, L.C., and Norden, C. (2015). Interkinetic Nuclear Migration Is Centrosome Independent and Ensures Apical Cell Division to Maintain Tissue Integrity. Developmental Cell 32, 203–219. https://doi.org/10.1016/j.devcel.2014.12.001.
3. Leung, L., Klopper, A.V., Grill, S.W., Harris, W.A., and Norden, C. (2011). Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development 138, 5003–5013. https://doi.org/10.1242/dev.071522.
4. Norden, C., Young, S., Link, B.A., and Harris, W.A. (2009). Actomyosin Is the Main Driver of Interkinetic Nuclear Migration in the Retina. Cell 138, 1195–1208. https://doi.org/10.1016/j.cell.2009.06.032.
5. Yanakieva, I., Erzberger, A., Matejčić, M., Modes, C.D., and Norden, C. (2019). Cell and tissue morphology determine actin-dependent nuclear migration mechanisms in neuroepithelia. Journal of Cell Biology 218, 3272–3289. https://doi.org/10.1083/jcb.201901077.
6. Amini, R., Bhatnagar, A., Schlüßler, R., Möllmert, S., Guck, J., and Norden, C. (2022). Amoeboid-like migration ensures correct horizontal cell layer formation in the developing vertebrate retina. eLife 11, e76408. https://doi.org/10.7554/eLife.76408.


 (6 votes)
(6 votes)