Delving into the complexity of hematopoietic stem cell genesis, fate, and developmental niches: novel insights from the zebrafish embryo and larva
Posted by Anne Schmidt, on 22 October 2025
Co-authored by Léa Torcq and Anne Schmidt
Before diving into the science and the path we took to reach our most recent publication in Development1, let me (Anne Schmidt, senior CNRS scientist, France) take a few lines to introduce the people behind the work.
The first author, Léa Torcq, studied mathematics and developmental biology. Léa began her PhD in October 2019, just a few months before the COVID pandemic. Our second key contributor is Catherine Vivier, our invaluable technician. The third and fourth members of our team are highly dedicated engineers from our platforms, Sandrine Schmutz and Yann Loe-Mie, who brought their excellent expertise in FACS-mediated cell sorting and bioinformatics, respectively. All three of them are staff members of the Pasteur Institute in Paris, France.
Léa and I will take turns sharing our exciting and fruitful collaboration aimed at tracking emerging and newly born hematopoietic stem cells to their implantation sites in developmental niches, using the zebrafish embryo and larva.
Anne: Our project began when — with my background in cellular and bio-membrane dynamics — I decided to investigate fundamental aspects of the cell biology of pre-hematopoietic stem cell emergence. This intriguing and unusual process, first visualized by Karima Kissa and Philippe Herbomel 15 years ago in the zebrafish embryo, is referred to as the Endothelial-to-Hematopoietic Transition or EHT2. To our knowledge, this unusual way of emerging from a flat tissue, where the cell bends outwardly from the aortic plane toward the sub-aortic space, appears to be specific to zebrafish (until it is observed in another tissue or species!).
While we began to unveil some fundamental aspects of these intriguing mechanics in 20183 (see our previous ‘Behind the Paper’ story), we questioned how the luminal membrane of these emerging cells is maintained throughout emergence and how it evolves after completion — with the key feature being the control of apico-basal polarity. Importantly, the evolution of this luminal membrane after release may influence the cell’s behavior, including its migration capacity (for example, if used as a membrane reservoir for cell locomotion), its signaling features (if recycled and/or degraded), and ultimately, its fate.
To tackle these ideas, I developed transgenic lines expressing a well-characterized apical marker, podocalyxin-l2, fused with eGFP (eGFP-podxl). Interestingly, using this line, EHT-undergoing cells imaged with confocal microscopy exhibited obvious asymmetric eGFP-podxl localization, with enrichment at the luminal membrane4. Astonishingly, the apical/luminal membrane is extremely dynamic (see Fig. 1) and eventually collapses into a pseudo-endocytic compartment after chasing engulfed intra-aortic fluid, which persists for several hours after release from the aortic floor (a post-EHT signature). This showed that apico-basal polarity is a key feature of EHT cells, maintaining a large apical domain until release, which is unconventional for a cell extruding from a tissue5. Perhaps this is the only way a cell can extrude from a flat tissue under strong mechanical tension (the tension exerted on the aortic wall by blood flow and its associated forces6), but in fact, it is not! Another outcome from our eGFP-podxl line unambiguously revealed another type of emergence dynamics, which showed no obvious apico-basal polarity. These emerging cells maintain a round shape (not resulting from recent mitosis), with endothelial neighbors crawling on their membrane facing the aortic fluid4. These two types of emerging cells, which we called EHT pol+ and EHT pol- cells (for polarized and unpolarized cells, based on podocalyxin localization), suggested that they may have different fates.
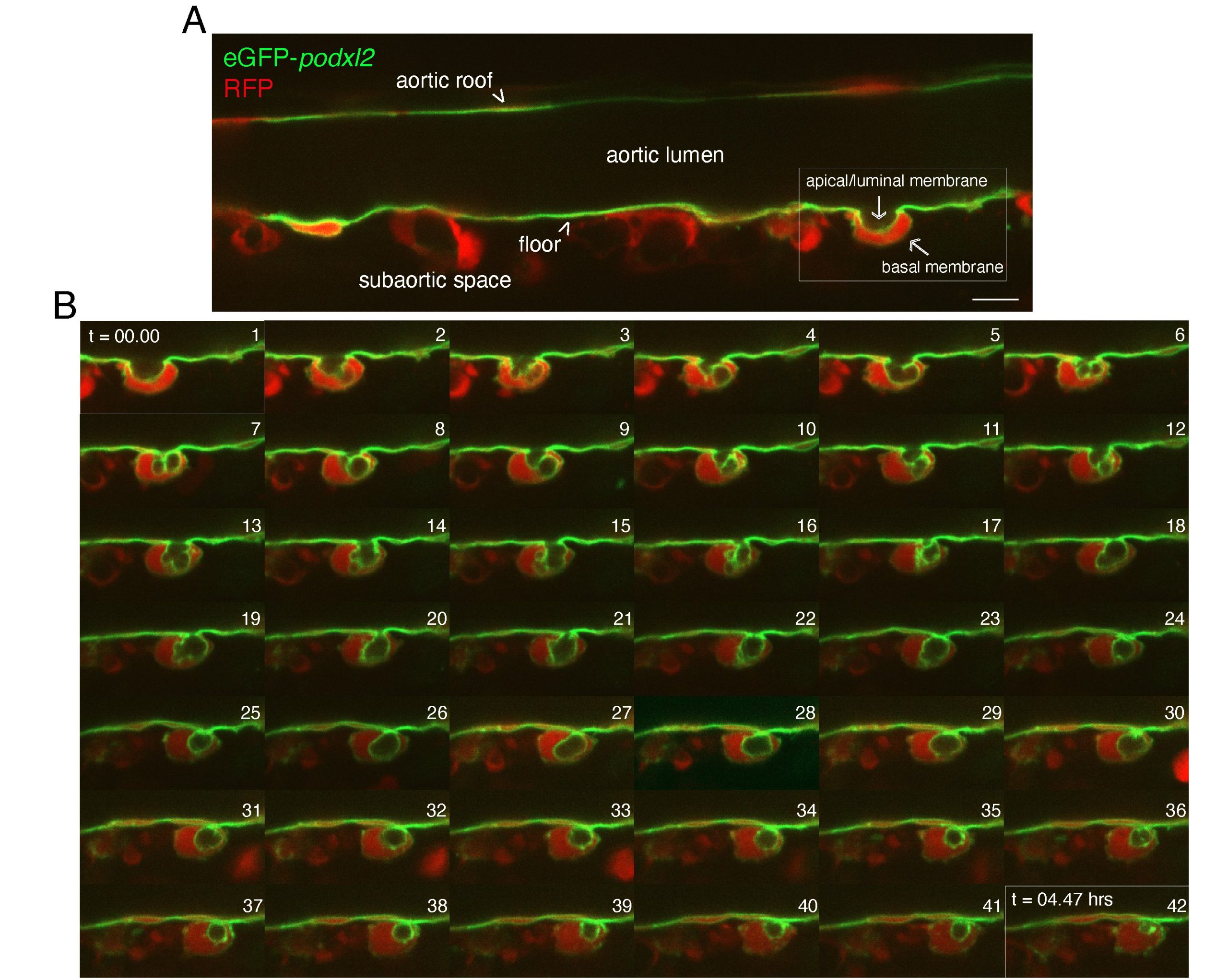
Figure 1: Series of z planes showing the dynamics of the EHT cell apical/luminal membrane labelled with eGFP-podocalyxinL2. Spinning disk confocal images obtained with Tg(kdrl:Gal4;UAS:RFP;4xNR:eGPF-podxl2) 50-55 hpf embryos (green: cellular membranes; red: cytosolic RFP). A, single z plane of a longitudinal section of the dorsal aorta (aortic lumen) showing one pre-hematopoietic stem or progenitor cell undergoing EHT (white delimited area on the right). Note the inward bending of the cell, toward the sub-aortic space. B, cropped views of single z planes extracted from a time-lapse sequence starting with the timing point visualized in the field delimited in (A). Images were acquired with 7 minutes intervals, from t=00.00 to t=04.47 hours as indicated in panels 1 and 42, respectively. Numbers 1 to 42 correspond to the progression of the time-lapse sequence throughout time. Note the enrichment of eGFP-podxl2 in the apical/luminal membrane as well as its remarkable dynamics (ex: compare panel 1 with panels 4, 9-12, 14-18). Note also the apparent regression of the apical/luminal membrane in panel 42, indicating that the cell has completed emergence from the aortic floor (we make the interpretation that the cell has detached from the floor in panel 30). Scale bar: 12 µm.
Besides characterizing junctional dynamics at the interface between EHT cells and endothelial neighbors, Léa’s aim was to tackle the question: are EHT pol+ and EHT pol- cells leading to different progenies? The idea was to set up single-cell photoconversion of EHT-undergoing cells, exploring the niches into which progenies establish throughout early larvae (an invaluable advantage of zebrafish, which develops quickly yet remains small) and characterizing their molecular signatures using single-cell RNAseq (sc-RNAseq).
Léa: I joined the lab as a Master’s student to work on this project in January 2019. I had previously studied EHT in Thierry Jaffredo’s lab, conducting work on self-organizing quail embryo explants in vitro. Although such models are fundamental to scientific discovery, I wanted to move toward more physiological, in vivo approaches. When I met Anne and she showed me the movies generated through live imaging of transgenic zebrafish embryos, I was instantly fascinated by this beautiful – both scientifically and aesthetically – approach. I also quickly discovered that Anne was a unique kind of senior researcher. She remains actively involved in performing and analyzing experiments at length with a combination of youthful drive for science with wise meticulousness, which convinced me to follow my initial internship with a PhD, with Anne as my advisor.
I was also drawn to this project because it gave me the opportunity to learn many different techniques, ranging from live imaging to scRNAseq and transgenesis. It came with challenges in optimizing and analyzing these diverse experiments, compounded by the COVID lockdown during most of 2020. Nevertheless, we persevered, and I was fortunate to receive invaluable help from several people, particularly our co-authors. Catherine taught me how to perform in situ hybridization and set up single molecule fluorescence in situ hybridization (smFISH), using RNAScope. Sandrine, from our institute’s cytometry platform, spent around 100 hours sorting cells for subsequent scRNAseq experiments. As for Yann, he originally helped set up the analysis pipeline for MARS-seq and provided guidance when I began training myself in scRNA-seq analysis.
Overall, we used nearly 400 embryos for photoconversion of single cells based on their morphology as they emerged from the aorta. Subsequently, larvae were used to track migration patterns and build precise lineage trees. We also index-sorted the progenies of 2,036 photoconverted cells and generated MARS-seq libraries from them. Separately, we used gata2b and cd41 reporter lines and 10X Chromium to generate a complementary scRNA-seq dataset of more than 30,000 cells, encompassing the whole hematopoietic lineage sorted by their niche of origin. Our main discovery was the differential fate of EHT pol+ and EHT pol- cells, with a bias regarding the lymphoid lineage. We identified different propensities to seed the thymus as well as different abilities to differentiate into T-lymphocytes. Moreover, our work contributes to the characterization of zebrafish hematopoietic cell types with new insights on the origin of some populations, like the ILC2-like and ILC3-like cells, never before observed at such early developmental stages.
Anne: Léa’s hard experimental and bioinformatic work has been extremely fruitful. From our single cell pipelines and their integration, we retrieved informative signatures of Hematopoietic Stem and Progenitor Cell (HSPC) populations. These included the transcription factor gata2b7 (which is upstream of runx1, a transcription factor essential for hematopoiesis8) and podocalyxin/cd34. Intriguingly, we found that in addition to embryonic HSPCs (eHSPCs) and other multipotent progenitors, gata2b is also expressed in sub-populations of ILC2-like cells enriched in the anterior/trunk region of the larvae, and of young eosinophils. We discovered that eosinophils possess the unique property of differentially expressing genes related to extracellular niche/matrix functions, including serine protease inhibitors of the spink2 family, timp4.2 (an inhibitor of metalloproteases), as well as one specific member of the MFAP4 locus. We then used these markers to investigate the localization of hematopoietic populations through whole-mount in situ hybridization. With Catherine’s expertise, we developed RNAscope applied to zebrafish. While whole-mount RNAscope had rarely been used for zebrafish embryos and larvae at that time – essentially because chromogenic and/or fluorescent in situ hybridization using long antisense nucleotide probes were routinely used and at relatively low cost –, it proved to be a great decision. Because it provided a high signal-to-noise ratio and sensitivity, RNAscope allowed us to investigate cells implanted in niches throughout the entire early larval body, including the pronephros region, which is challenging because it requires deep penetration of probes and low background (see our recent technical paper 9).
With the timp4.2 marker highly expressed in eosinophils, we found an intriguing accumulation of cells almost exclusively in the most anterior region of the pronephros, in the 5 dpf larva. These cells, with a maximum of 15 per animal in that region (on average more than in the trunk and the Caudal Hematopoietic Tissue (CHT)), also faintly express eGFP driven by an enhancer of the hematopoietic transcription factor runx1, confirming their hematopoietic origin (see Fig. 2 and Movie 1). This pointed to sub-compartmentation of the pronephros niche. Currently, we do not know if these cells, presumably eosinophils (or progenitors), home there for maturation and/or if they contribute to building a sub-niche hosting specific hematopoietic cell subtypes. Anyhow, these results highlight the functional complexity of the developing pronephros niche and point to the importance of investigating micro-environmental properties supporting the differentiation and/or maintenance of specific hematopoietic populations.
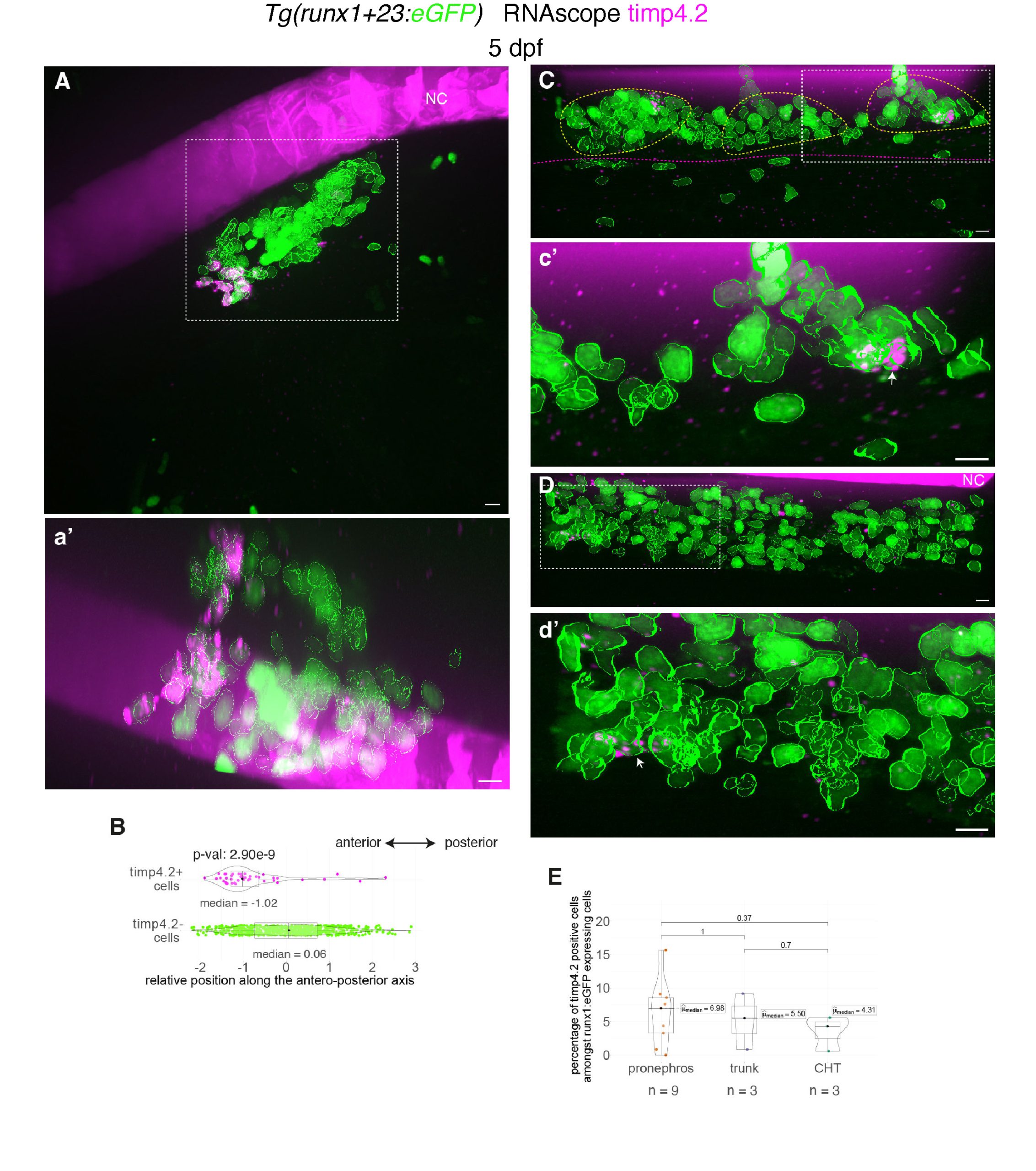
Figure 2: Whole-mount in situ hybridization revealing timp4.2 mRNA expression in hematopoietic and vascular cells using RNAscope. A, C, D, Representative images (Imaris 3D-rendering) of RNAscope ISH for timp4.2 (magenta spots) in 5 dpf Tg(runx1+23:eGFP) larvae. Images show the pronephros region (A, see also Movie 1), the posterior trunk region (C, above the elongated yolk) and the CHT (D). a’, c’, d’ are magnifications of regions outlined with white dashed boxes in (A, C, D), respectively. eGFP positive hematopoietic cells were segmented (green contours). White arrows point at timp4.2 positive hematopoietic cells. The sub-aortic clusters are delimited by yellow dashed lines and the gut by magenta dashed lines. B, Relative position of eGFP positive cells along the antero-posterior axis of the pronephros (n=681 timp4.2– cells, n=46 timp4.2+ cells). E, Percentage of eGFP positive hematopoietic cells expressing timp4.2, n=6 larvae for pronephros, n=3 for trunk and CHT regions. (B, E) Two-sided Wilcoxon tests. NC: notochord. Scale bars: 10 µm.
Movie 1: 3D visualization of RNAscope in situ hybridizations for timp4.2. Timp4.2 (in magenta) in the pronephros region of Tg(runx1+23:eGFP) 5 dpf larvae, 3 representative replicates are shown. Bottom row shows magnifications of the top row. Hematopoietic cells in the pronephros are delineated (green contours). Scale bars: 10 µm.
Finally, the most unexpected results came when using the gata2b probe. We detected strong expression of this transcription factor in endothelial cells of the supra-intestinal artery (SIA), a small vessel located just above the intestinal tract and beneath the posterior cardinal vein (for detailed anatomy, see Isogai et al10). We obtained these results in March 2023, more than 2 years ago and about a year and a half before submitting our paper to Development. Importantly, our images unambiguously showed that not only do SIA endothelial cells express gata2b, but so do other cells in their direct vicinity, even contacting the SIA wall. These cells also express eGFP driven by the vascular kdrl promoter, and it appeared that many of them express eGFP at levels comparable to SIA endothelial cells.
Léa: When we realized this, we considered that the SIA region might not only be a niche for hematopoietic stem and progenitor cells and more differentiated cells whose ancestors emerged from the dorsal aorta days before (e.g., ILC-like cells with immune functions in the gut11), but that these cells might also derive directly from the SIA wall itself! To reinforce our results, I quantified the number of gata2b-positive cells in the direct surroundings of the SIA as well as near the dorsal aorta, showing that the SIA region is significantly enriched in gata2b cells compared to the dorsal aorta. After 3D segmentation of the cells with Imaris, I quantified their eGFP signals (driven by the kdrl promoter) and found that gata2b-positive cells near the SIA express comparable levels of eGFP to SIA endothelial cells (with no such cells around the dorsal aorta). This suggests they are relatively newly born cells whose eGFPcontent has not been diluted by division cycles, reinforcing the idea that they may originate from the SIA endothelium.
Anne: All this evidence supports the hypothesis that the SIA may be hemogenic. Due to the constant movement of the gut beneath the SIA, we struggled to provide high quality time-lapse sequences for our paper in Development (even acquiring a single complete z-stack was difficult). However, we obtained discontinuous images over relatively short periods of up to 2 hours that strongly suggest emergence from the SIA wall (see Fig. 3). Importantly, cells undergoing apparent emergence remained very ‘sticky’ to the SIA wall, making it difficult to confirm they fully completed EHT. Our results clearly demonstrate that the SIA region is at least a niche hosting HSPCs and suggest that these may be born from this small artery. Hence, the SIA may be hemogenic, a potential novel finding requiring further validation. As discussed in our Development paper1, this validation will require characterizing the SIA hemogenic endothelium and the fate of the derived EHT cells at the single-cell level.
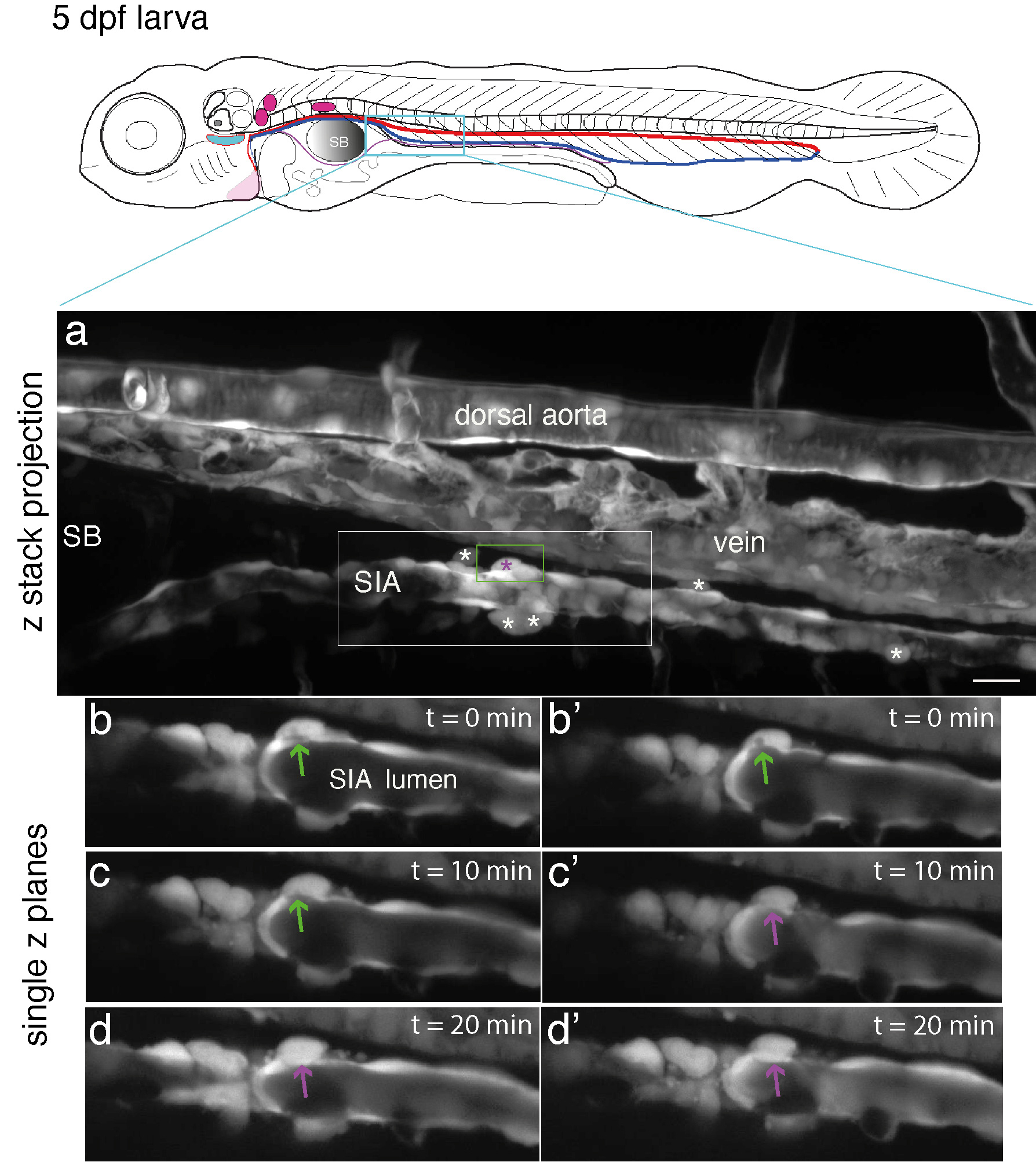
Figure 3: Evidence of emergence from the SIA endothelium. Top panel: schematic representation of a 5 dpf larva (reproduced with modifications from Schmidt, 2022, doi:10.7554/eLife.64835); red line = aorta, blue line = vein, magenta line = SIA. (a – d’), spinning disk confocal microscopy of a 5 dpf Tg(kdrl:eGFP) zebrafish larva, in the upper part of the trunk region (delimited in the cyan box of the upper cartoon). Panel a (z projection), the upper part of the trunk region encompassing the dorsal aorta, the posterior cardinal vein, and the SIA with the latter passing beneath the swim bladder (SB, on the left side of the image). White and magenta asterisks indicate cells expressing eGFP at apparently comparable level than SIA endothelial cells and that are contacting the SIA wall (note that no such cells are in contact with the ventral floor of the dorsal aorta). (b – d’), single z planes extracted from the z stack projected in (a), with images magnified from the region in (a) delimited by the white rectangle and showing the cell surrounded by the green rectangle in (a) and undergoing emergence between 0 min (b, b’), 10 min (c, c’), and 20 min (d, d’). Panels (b, b’), (c, c’), and (d, d’) are images separated by 1 mm depth in z. Green arrows point at the connection of the emerging cell with the aortic lumen; magenta arrows point at the disappearance of this connection, which suggests completion of the emergence. Scale bar: 20 µm.
This story behind our paper in Development summarizes an exciting research journey that led to several previously undescribed findings. This was made possible by assembling a team of passionate and efficient people and pushing forward the resolution of our analyses, including technically demanding single cell photoconversion, multiple single-cell RNAseq approaches, and powerful smFISH technology using a new generation of small, highly specific probes (we’ve significantly contributed to increasing the number of zebrafish hematopoietic probes in the ACD catalogue!).
Finally, we are convinced that our work opens new avenues for exciting future discoveries in the fields of hematopoietic stem cells and vascular biology.
References
1. Torcq, L., Vivier, C., Schmutz, S., Loe-Mie, Y., and Schmidt, A.A. (2025). Single-cell and in situ spatial analyses reveal the diversity of newly born hematopoietic stem cells and of their niches. Development 152, dev204454. https://doi.org/10.1242/dev.204454.
2. Kissa, K., and Herbomel, P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115. https://doi.org/10.1038/nature08761.
3. Lancino, M., Majello, S., Herbert, S., De Chaumont, F., Tinevez, J.-Y., Olivo-Marin, J.-C., Herbomel, P., and Schmidt, A. (2018). Anisotropic organization of circumferential actomyosin characterizes hematopoietic stem cells emergence in the zebrafish. Elife 7, e37355. https://doi.org/10.7554/eLife.37355.
4. Torcq, L., Majello, S., Vivier, C., and Schmidt, A.A. (2024). Tuning apicobasal polarity and junctional recycling in the hemogenic endothelium orchestrates the morphodynamic complexity of emerging pre-hematopoietic stem cells. Elife 12, RP91429. https://doi.org/10.7554/eLife.91429.
5. Staneva, R., and Levayer, R. (2023). Cell polarity and extrusion: How to polarize extrusion and extrude misspolarized cells? Curr Top Dev Biol 154, 131–167. https://doi.org/10.1016/bs.ctdb.2023.02.010.
6. Campinho, P., Vilfan, A., and Vermot, J. (2020). Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior. Front Physiol 11, 552. https://doi.org/10.3389/fphys.2020.00552.
7. Butko, E., Distel, M., Pouget, C., Weijts, B., Kobayashi, I., Ng, K., Mosimann, C., Poulain, F.E., McPherson, A., Ni, C.-W., et al. (2015). Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142, 1050–1061. https://doi.org/10.1242/dev.119180.
8. Gao, L., Tober, J., Gao, P., Chen, C., Tan, K., and Speck, N.A. (2018). RUNX1 and the endothelial origin of blood. Exp. Hematol. 68, 2–9. https://doi.org/10.1016/j.exphem.2018.10.009.
9. Torcq, L., and Schmidt, A. (2025). Single Molecule Fluorescence In Situ Hybridization Using RNAscope to Study Hematopoietic and Vascular Interactions in the Zebrafish Embryo and Larva. BIO-PROTOCOL 15. https://doi.org/10.21769/BioProtoc.5269.
10. Isogai, S., Horiguchi, M., and Weinstein, B.M. (2001). The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Developmental Biology 230, 278–301. https://doi.org/10.1006/dbio.2000.9995.
11. Hernández, P.P., Strzelecka, P.M., Athanasiadis, E.I., Hall, D., Robalo, A.F., Collins, C.M., Boudinot, P., Levraud, J.-P., and Cvejic, A. (2018). Single-cell transcriptional analysis reveals ILC-like cells in zebrafish. Sci Immunol 3. https://doi.org/10.1126/sciimmunol.aau5265.


 (No Ratings Yet)
(No Ratings Yet)