Engineering morphogenesis using self-organized embodiment
Posted by SDuranNebreda, on 20 January 2021
During their journey from zygote to adult, embryos experience several symmetry breaking processes. Structures which are not isotropic (equal in all directions) are formed, creating the inside-out axis, forward-backwards axis, etc. Each of these patterns increases the information required to describe a multicellular system. As Maynard Smith put it: sometimes the “extra” information is stamped by maternal cues (gradients) and other times it emerges from self-organized processes. Decades of research have accrued a lot evidence for self-organized processes in development, from the theoretical to the experimental. More recently, synthetic biology has provided an avenue for enquiry by allowing us to create in vivo and de novo patterning mechanisms.
In our recent publication we take an engineering approach by inducing a symmetry breaking process in growing bacterial colonies. Without external factors like lack of nutrients or chemoattractants, E. coli colonies develop with remarkable symmetry into circular shapes. Their propagation front is isotropic and homogenizing diffusion is the leading morphogenetic drive, erasing any patterns in cellular density.
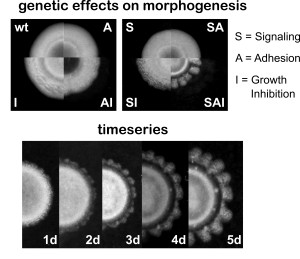
We induced pattern formation using four synthetic genes relating to adhesion, signaling and growth inhibition. These would make cells stop dividing and attach to one another, but only once a certain local cellular density threshold had been surpassed. With this we hoped that differences in cell density would be amplified with short range activation and long range inhibition, and that the patterns in space would be preserved from diffusion through adhesion.
When we put these elements together in growing colonies, we observe a remarkable symmetry breaking process. Colonies now develop into flower-like shapes with “petals” forming at a characteristic scale (constant wavelength). Regions with increased cell density inhibit the growth of neighboring cells and delay front propagation. This emergent pattern is not found in any other possible combination of our synthetic genes.
We believe these is just a first step into developing an engineering research program of artificial developmental processes. Using mechanical processes inherent to cellular embodiment allows us to make robust patterns that emerge from many interacting agents. Hopefully, armed with bioengineering tools we will gain a better grasp on the sometimes counter intuitive emergent interactions that create order in real developing embryos.


 (5 votes)
(5 votes)