Gene editing stem cells with CRISPR could help understand brain tumours
Posted by JustineCRUK, on 14 February 2017
 This report written by Justine Alford and highlighting a recent Development paper originally appeared on the CRUK Science Blog.
This report written by Justine Alford and highlighting a recent Development paper originally appeared on the CRUK Science Blog.
Over the past 12 months, the acronym CRISPR has been popping up in science news left, right and centre. And for good reason.
Hailed as a revolution in genetic engineering, this molecular toolbox lets researchers make remarkably precise changes to DNA. By observing the consequences of these alterations on cells, such as how they look or behave, scientists can begin to work out what certain genes do.
“CRISPR has completely transformed the landscape for how we study gene function,” says Dr Steven Pollard, one of our brain tumour experts from Edinburgh’s MRC Centre for Regenerative Medicine. “It’s opening up the human genome for us to be able to do what we want genetically.”
And because cancer is a disease of faulty genes, CRISPR has huge potential for studying a raft of different types of cancer.
Now, for the first time, a team of scientists led by Pollard has succeeded in using CRISPR to change genes in specialised neural stem cells, which are thought to play a role in how the most common type of brain tumour, glioblastoma, grows.
This important work, published in the journal Development, lays the foundations for future, more detailed investigations. But firstly, what are these neural stem cells that scientists are tinkering with, and why are they so important?
The stars of the brain
If you’re unfamiliar with stem cells, we all begin our lives as a small bundle of them in the womb. Like the shape-shifting Mystique from X-Men, these amazing cells can change their appearance, morphing into every specialist cell in the body.
In our brains, a type of stem cell – called a neural stem cell – divides to become the complex mixture of highly specialised cells forming our ‘grey matter’, including cells called astrocytes. These star-shaped cells play incredibly important roles in the brain, offering protection for other cells and repairing those that become damaged.
During the transformation from neural stem cell into an astrocyte, the dividing cells can make a genetic ‘spelling mistake’, leading to a tumour made up of rogue astrocytes.
But some of the tumour cells become ‘locked’ in a neural stem cell state and continue to grow uncontrollably, never becoming specialised. These haywire cells, called glioblastoma stem cells, are thought to be important in fuelling and maintaining brain tumour growth.
And the similarities between the glioblastoma stem cells and neural stem cells means that researchers can study genes in neural stem cells to understand more about how glioblastomas form.
By introducing deliberate genetic mistakes into the neural stem cells’ DNA, scientists can track the role they may play in cancer. And thanks to the work by Pollard and his team, they now how a range of powerful new approaches to do this, all built upon CRISPR.
Scissors and homing devices
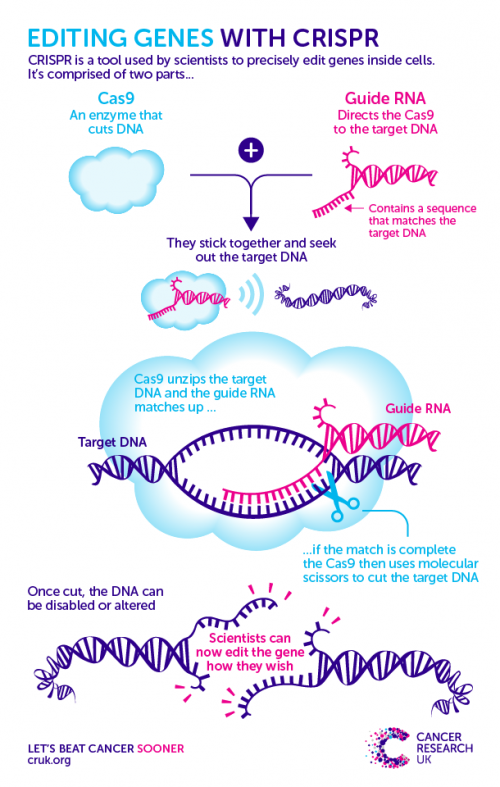
CRISPR is a two-piece toolkit that uses a homing device to seek out a specific region of DNA, and a pair of ‘molecular scissors’ to make a precise snip across the strands.
The cut DNA is then flagged to the cell’s own DNA repair system, which galvanises into action to sew the broken strands back together. Scientists then sneakily trick this system into making a mistake by providing a repair template that has a fault in it.
After this error is unwittingly copied into the DNA, scientists can track the consequences of this genetic change on the cell. Check out the graphic below to see how it works.
“Most groups have been using CRISPR to make random changes to genes, which is its simplest use,” Pollard explains. But the team’s latest study, he says, was focused on more sophisticated and precise changes made to neural stem cells in the lab.
For example, by stitching a fluorescent marker onto the molecule produced by a gene called SOX2, the scientists were able to track the journey that molecule takes around the cell. Precisely following molecules in this way could help researchers understand the role they play in cancer and find new targets for drugs.
“The obvious next step is finding out if we can use the same technology in cells taken from patients’ brain tumours,” says Pollard. By making important molecules glow inside these cells in the lab, Pollard believes they could “see which drugs are important in destroying that fluorescence”.
Making headway
After proving the editing prowess of CRISPR in neural stem cells, the scientists then moved on to their next challenge: could they deliberately introduce faults in genes already known to drive brain tumours?
The team focused its attention on 2 different genes. The first, p53, protects cells from becoming cancerous. It’s faulty in more than half of human tumours, including many brain tumours.
The second is a gene called H3F3A, which is commonly faulty in childhood glioblastomas. H3F3A helps package up our DNA into chromosomes.
The team managed to make faulty versions of both of these genes in human neural stem cells. And when they studied the cells harbouring the faulty version of p53 in the lab, they found that they divided faster – a hallmark of cancer.
While it’s still early days, this study has important implications for brain tumour research and therefore ultimately for patients. By demonstrating that CRISPR can successfully be used to create and study faulty genes, the team has opened the door for more in-depth research into what genes play a role in these brain tumours.
“It’s proof of principle that it works very efficiently,” says Pollard of the technique. “It’s a toolbox to show that, in the future, we can target other genes to study them and manipulate them in diverse ways. This will help us to understand their function and reveal how to manipulate them with new drugs.”
And by using CRISPR to understand how brain tumours grow, this could lead to more targeted treatments.
Although significant progress has been made in cancer medicine, with survival across all cancers doubling over the past 40 years, poor survival is a shared feature among brain tumours, making them one of our top priorities.
It’s clear that we urgently need to do more. And kick starting a greater understanding of brain tumours is needed, which is exactly what this study does.


 (2 votes)
(2 votes)