Going out on a limb to study organ growth
Posted by Alberto Rosello-Diez, on 2 August 2018
Alexandra Joyner and Alberto Roselló-Díez tell us the story behind their recent paper in PLoS Biology1.
Today we have tried a new experiment (we cannot help it). Instead of elaborating too much on the scientific aspect of our recent paper about the control of organ growth in mammals1, we decided to tell the personal aspect of it. It’s a story about perseverance, collaboration and serendipity, and we hope it will be especially encouraging for young trainees striving to find their niche. Here we go:
Alex: One of the big mysteries in developmental biology is how the robustness of our body plans is achieved, perhaps best exemplified by the near equal lengths of our left and right limbs. Alberto started devising a new experimental approach to this fascinating question with a comprehensive review written by Cliff Tabin’s group in hand2, but there appeared to be limited investigations on which to base his work.
Alberto: Indeed! That’s how this story started. When I was finishing my PhD in Miguel Torres’s lab in Spain, Miguel had a great idea for a departmental retreat. Everyone had to think about a wild project, a particularly difficult question they would like to address if they had the resources to do it. Long story short, I presented my idea that while limb growth was mainly autonomous, perhaps the fine-tuning of limb size involved some kind of limb-limb crosstalk. My proposed approach involved complicated ways of achieving unilateral growth manipulation in a variety of vertebrate and invertebrate models, in order to study the potential recovery of symmetry. Frankly, my approaches were hardly viable, but then I got a suggestion from a brilliant colleague who was also doing his PhD at the time, my friend Juanma González Rosa. He suggested an elegant way of achieving unilateral manipulation: to use mouse Cre lines driven by the regulatory region of some of the known left-specific genes that operate during early development. And that was the seed of the project. We came back to our labs, and while I was writing my thesis, a review article from the great developmental biologist Lewis Wolpert revisited the topic of limb symmetry as one of the remaining mysteries in developmental biology3. This obviously bolstered my interest in the topic, and as soon as I graduated and submitted the revisions of one of our papers, I spent the following 3-4 weeks reading, formulating hypotheses and writing an experimental plan. Finding the left-specific Cre line was no easy task, because the people who had generated them were not interested in limb development, and their papers did not mention the expression in the limbs. Fortunately, they were so kind as to respond to the out-of-the-blue request of a young PhD, and even sent me pictures of staining in the limbs (thank you again, Drs Martin, Shiratori and Hamada). And that’s how I found the Pitx2-Cre driver that would be key for our studies4, and that Alex would later import from Japan even before I arrived to the lab. However, the Cre was expressed in the very early embryo, marking the left lateral late mesoderm from that stage onwards, so other genetic components were necessary to enable manipulation specifically at the critical period of limb length establishment. According to Wolpert, the problem had to be addressed at the level of the growth plate of the elongating bones, so I devised an intersectional strategy to restrict any cellular modification of interest to the growth plates of the left long bones. The new transgene I designed depended on the coincidence of two drivers, Cre and (r)tTA, to activate expression of a growth altering protein in a given cell population. The idea became to restrict expression of the protein using Pitx2-Cre and a cartilage specific rtTA in combination with the new transgene. This strategy would provide not only exquisite spatial control, but also inducibility and reversibility, as rtTA requires Doxycycline to be active.
It was March 2011 when I went back to the lab after my post-PhD reflection period. I was full of energy and determined to find a lab in which I could develop my idea. My hypothesis at the time was that the nervous system could be involved in communicating the left with the right limb, and maybe even comparing their lengths, so I ideally had to find a lab with expertise in limb and nervous system development, and the resources and experience to generate the complex genetic models the project required. I thought it would be difficult, but in a curious twist of fate, the opportunity quite literally presented itself when Alex visited our institute to present a fascinating story about nerve-released sonic hedgehog (SHH) having an important role in the fate of the stem cells of the hair bulge5. Alex had published several studies on limb development before, was an expert in nervous system development and function (especially the cerebellum), and was developing several models of recovery after organ injury, so Miguel kindly introduced me to her during their allocated meeting time.
Alex: It was unusual to have a recently graduated student on the schedule of meeting Miguel sent me, but then Alberto is an unusual scientist! After telling me about his exciting PhD limb research, Alberto proceeded to tell me about what he wanted to do for his postdoctoral research. Loving a genetic challenging in mice, I was very taken by the elegant approach Alberto had come up with to study one of the most basic and fascinating questions in development – how symmetry is attained. He visited our lab at MSKCC in New York soon after, and there was unanimous excitement to have Alberto join our group and start a new area of research.
Alberto: The excitement was reciprocal! I chose to join the lab because I perceived an atmosphere of constructive feedback and criticism during my interview, and that’s exactly what I needed for this type of project that ventured in uncharted waters. But, to be honest, things were everything but easy when I moved to New York in June 2012. First, generating the mouse lines would be quite resource demanding, so we had to choose our experiments carefully. Second, one of my experiments was more complicated than expected. I decided to try to characterise with high precision the left-right limb asymmetry at several stages of mouse development while I was building the transgene constructs, and also correlate it with the presence or absence of innervation near the growth plate, and determine how it was affected after pharmacological manipulation of candidate neurotransmitter response pathways. The precision required made the process extremely painstaking, and the results of the pharmacological manipulation were inconclusive, so when the first mice of my intersectional model were available, roughly a year after I started in Alex’s lab, we decided I would fully dedicate my time to characterise the brand-new mice. A third challenge then arose, the double-conditional allele (which I eagerly called Dragon, standing for Dox-controlled and Recombinase-Activated Gene-OverexpressiON) was unexpectedly not active in most of the cells that were supposed to express the transgene, seemingly pushing us back to square 1.
Alex: Yes, the first year was quite difficult for us but proved to me that Alberto had the resourcefulness to make the project work. Fortunately, in another twist of fate, I got in contact with Dr. Hongkui Zeng whom I had met at the Allen Institute and learned that the Tet-responsive element becomes easily inactivated in the locus we used, but that she had identified an intergenic region after extensive screening that supports (r)tTA-driven gene expression6. She had named the locus TIGhtly-REgulated or Tigre, the Spanish word for tiger (quite fitting, as Alberto is from Spain). Intriguingly, Dr. Zeng’s group was also working on a Cre- and (r)tTA-dependent intersectional gain-of-function strategy7, so we teamed up with them to modify their system and adapt it to our needs, and they generously offered to perform the embryonic stem cell manipulations.
Alberto: Yes, we quite literally found the crouching Tiger for our hidden Dragon! This collaboration was undoubtedly key to finishing the project in a timely manner, but would still require half a year to make the mice. Therefore, while our Tigre-Dragon mice were being generated, we also developed a fast way of using Pitx2-Cre to induce unilateral cell death in the limb, with which we found that the tissues surrounding the long bones can modulate bone growth8. This result made us wonder if there was communication between other tissues and the bones, and if it could work in the opposite direction. Spoiler alert: it does!
Alex: That’s right! When Alberto was finally able to analyse our Tigre-Dragon mice, we were surprised to see that the mice did not show any obvious asymmetry despite having more than 50% of their chondrocytes arrested in the left hindlimb. Long story short, we found that two compensatory mechanisms accounted for symmetry maintenance: local hyper-proliferation of the undamaged cells, almost perfectly tuned to make up for the arrested cells, and more surprisingly, a systemic growth reduction that affected all limbs and the whole embryo in general. Although the systemic effect was subtle, we were quite excited about it, because to our knowledge it was the first time that this phenomenon was shown in an organism other than developing insects.
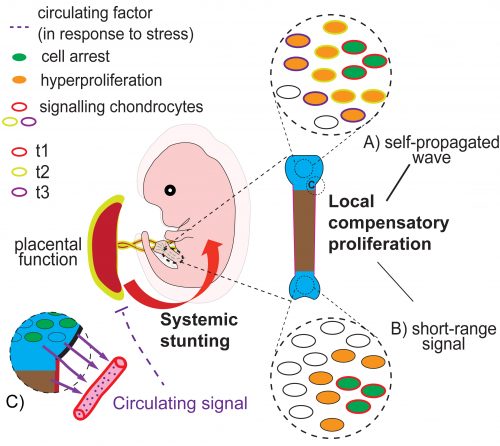
Alberto: Yes, that was a very positive surprise. I feel lucky that we discovered new fundamental mechanisms of growth regulation right when I was going to start my own lab. We would have liked to uncover more about the molecular mechanisms underlying our observations (and so did the reviewers), but to be fair that took over 30 years in Drosophila, so I guess it will be a long and difficult quest!
Alex: Alberto is starting the next stage of his career as an independent investigator with a wealth of interesting results and transgenic mice as a strong foundation for a lifetime’s worth of exciting projects for his lab. His perseverance has paid off in spades, along with his careful evaluation of every experimental design and result, an openness to collaborate, a thirst for charting new ground and of course a little bit of luck.
Alberto: Don’t forget the mentor that kept me on track!
References
1 Rosello-Diez, A., Madisen, L., Bastide, S., Zeng, H. & Joyner, A. L. Cell-nonautonomous local and systemic responses to cell arrest enable long-bone catch-up growth in developing mice. PLoS Biol 16, e2005086, doi:10.1371/journal.pbio.2005086 (2018).
2 Allard, P. & Tabin, C. J. Achieving bilateral symmetry during vertebrate limb development. Semin Cell Dev Biol 20, 479-484, doi:10.1016/j.semcdb.2008.10.011 (2009).
3 Wolpert, L. Arms and the man: the problem of symmetric growth. PLoS Biol 8, doi:10.1371/journal.pbio.1000477 (2010).
4 Shiratori, H., Yashiro, K., Shen, M. M. & Hamada, H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development 133, 3015-3025, doi:10.1242/dev.02470 (2006).
5 Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. & Joyner, A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552-565, doi:10.1016/j.stem.2011.02.021 (2011).
6 Zeng, H. et al. An inducible and reversible mouse genetic rescue system. PLoS Genet 4, e1000069, doi:10.1371/journal.pgen.1000069 (2008).
7 Madisen, L. et al. Transgenic Mice for Intersectional Targeting of Neural Sensors and Effectors with High Specificity and Performance. Neuron 85, 942-958, doi:10.1016/j.neuron.2015.02.022 (2015).
8 Rosello-Diez, A., Stephen, D. & Joyner, A. L. Altered paracrine signaling from the injured knee joint impairs postnatal long bone growth. Elife 6, doi:10.7554/eLife.27210 (2017).


 (1 votes)
(1 votes)