Growing a body, one tiny tug at a time
Posted by Knowable Magazine, on 24 February 2020
From Knowable Magazine’s Special Report: Building Bodies. For an introduction to the series see Eva Emerson and Rosie Mestel’s Node post.
For decades, genetics and biochemistry have formed the bedrock of developmental biology. But it turns out that physical forces — the way cells push, pull and squeeze each other — play a huge role, too.
By David Levin
In every parent’s life there comes a singular moment, a knife edge when the world bisects neatly into two parts. There’s life before, and life after. Mine split irreversibly on a frigid December morning, just past 4 a.m., as my son took his first breath and sent me reeling over the threshold of fatherhood, ecstatic and teary-eyed.
Witnessing the birth of my first child opened up whole new avenues of developmental biology for me, as a science writer, to ponder. How could our little miracle, with his wriggling legs, searching gaze, tiny, grasping hands, emerge from a minuscule ball of cells? The basic concept has been drilled into our brains since we were kids: From the union of sperm and egg comes an embryo. But as that egg divides and grows, how does the structure of a body come to be?
It’s a doozy of a question. For more than half a century, the go-to answer has been DNA and biochemistry: Nearly every aspect of development, researchers thought, could be brought on by triggering the right genes at the right times or adding the right chemical signal, creating a cascade of precise transformations. Over the past few decades, though, a growing body of work has cast a spotlight on another starring role in this show. Physical and mechanical forces — things that pull and stretch and twist and squish cells — act alongside genes, and play a major role in everything from the formation of organs to the structure of our limbs to the onset of disease. Serious conditions like certain types of muscular dystrophy and cancer, which seem entirely disparate on the surface, may result from a breakdown of force-sensing mechanisms in cells.
The idea isn’t exactly new. “If you get books on science from the 1890s to the 1910s, everything’s explained mechanically,” says bioengineer and cell biologist Don Ingber, the founding director of Harvard’s Wyss Institute and coauthor of a 2013 overview of the role of mechanics in cell development in the Annual Review of Cell and Developmental Biology. “Then chemistry came in, genetics came in, and the baby was thrown out with the bathwater.”
Armed with technology that can probe and measure forces at a cellular level, more scientists are starting to return to these century-old concepts — and according to Ingber, it’s becoming increasingly clear that physical forces and mechanics are a crucial part of developmental biology.
A key 2006 study in the journal Cell showed just how dramatically force can change cell function. When adult stem cells grew on a soft surface, the paper reported, they started to form something that looked like developing neurons — but when they grew on a hard substrate, they instead took on the shape of osteoblasts, the precursor cells to bone.
“If the cell is round and floppy, or spread out wide and pulled taut, you get totally different functions,” Ingber says. In other words, the forces that a stem cell “feels” on its surface may determine what sort of tissue it will ultimately become.
It’s still not obvious how those minute forces help to shape the formation of all of a body’s requisite parts, however. What signals do they provide that drive the development of limbs? Of organs? Of a brain that harbors thoughts and feelings and fears?
“Those are the mysteries still being uncovered,” says Celeste Nelson, a developmental bioengineer at Princeton University who studies developmental mechanics and coauthored an article on aspects of the topic in the Annual Review of Biomedical Engineering. “The best guess is that yes, mechanical force is important and mechanical stresses are essential. But what exact signals are being sent, and how those are being translated into alterations in cells at these early stages, is still a bit unclear. This is what half of my lab is working on right now.”
Sensing force
The clues to how this all works, Nelson says, might be revealed by studying how cells sense force in the first place — and there are a few different ways a cell can pull this off.
It can use stretch-sensitive ion channels, tiny valves in the cell membrane that let charged atoms like calcium flow in and out, triggering a variety of biochemical signals. Or it can exploit cell-cell junctions, points where certain types of cells are “spot-welded” to their neighbors by globs of proteins.
It could also be leaning on well-studied structures that sense forces during development: clusters of proteins called focal adhesions.
These adhesions anchor cells to the extracellular matrix, the fibrous scaffold that surrounds them. And as that scaffold pulls or stretches a focal adhesion, two things happen: First, proteins inside the adhesion trigger a flood of biochemical signals that alter structural functions inside the cell. Second, and more profoundly, the focal adhesion tugs on a vast structural web inside of cells, called the cytoskeleton.
This mesh acts like a series of I beams and guy wires, supporting the cell membrane and lending the cell its overall shape and form. Crucially, it also connects the focal adhesions to the cell’s nucleus, where its DNA is stored. And that, says Jan Lammerding, a biomedical engineer at Cornell University, could be one of the keys to how physical force influences a cell’s fate.
Any force on the cell’s surface, he says, may be transmitted through the cytoskeleton and passed on to the nucleus, which itself will be stretched or twisted. As the nucleus is squished around, some genes inside it will be newly exposed, and become more active. Others will be suddenly hidden, making them less active. In effect, it may be possible to modify the genetic function of a cell just by yanking on its nucleus.
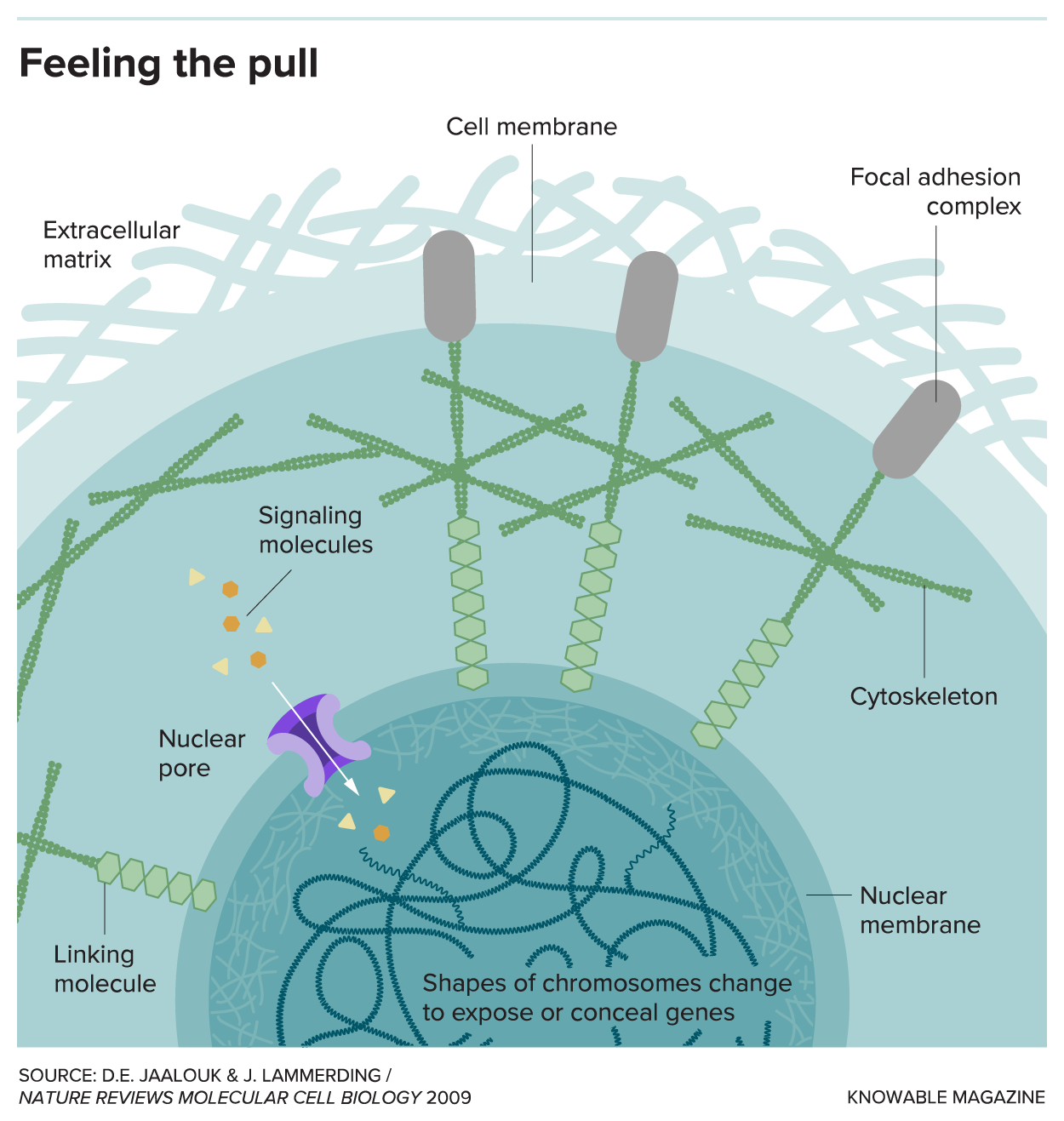
Those changes go both ways. When the nucleus senses force, proteins inside it called Lamin A pile together en masse, making the organelle stiffer. As the nucleus hardens, it can alter the activity of genes inside it, and some of these changes can set off a chain of events that makes the extracellular matrix stiffer. The matrix, in turn, exerts even stronger forces back into the cell.
This constant push and pull between a cell and its matrix creates a powerful feedback loop. Any changes in the cell will trigger changes in the matrix; any changes in the matrix will trigger even more changes in the cell, and with each iteration, the cell is steered further and further toward its final incarnation, be it a muscle cell, nerve cell, liver cell or any other cell in the body. Sheets or clusters of those differentiated cells expand and fold and, steered by mechanical forces and other inputs, come to sculpt whole organs.
Matters of the heart
In truly poetic fashion, the first organ to emerge in a human embryo is the heart. It starts as a loosely organized tube, explains Dennis Discher, a bioengineer at the University of Pennsylvania, and as it grows, the cells within it harden gradually more each day. That’s a fact cynics everywhere will relish. But according to a 2016 study by Discher and his lab, this hardening may be responsible for the fact that the heart beats at all.
Inside the nascent organ, molecules of Lamin A collect inside the nucleus of cells called cardiac myocytes, causing them to stiffen and elongate. At the same time, the cells exude an increasing amount of collagen, a tough, stringy protein that hardens the extracellular matrix around them. The forces each exerts on the other progressively increase, like a game of cellular tug-of-war, until they reach a critical threshold, Discher says.
As the forces hit that breaking point, a cell somewhere in the mix will spontaneously contract, yank on its neighbors and cause them to contract as well. The resulting chain reaction ripples out among the cardiac cells, triggering an initial heartbeat. “As soon as you get one contracting a certain amount, if it’s connected to all the other cells around it mechanically, then it’s going to set off a reaction called a peristaltic wave that goes on down the line,” Discher says.
Thanks to the constant feedback between forces inside and outside the cells, every pulse of that wave will increase the stiffness of the cardiac cells and the scaffold they sit in, progressively steering the cells’ development. Gradually, that and other inputs will lead them to morph into the specialized, four-chambered muscle that sustains us throughout our lives.
A similar process may happen as lungs and other organs develop. As cells there mature and tissues stiffen, ones that formerly grew in flat sheets (epithelial cells) change their arrangement. This shuffling exerts new forces that can cause the sheets to buckle and fold, like a pair of hands pushing on a tablecloth. Those folds start to rise out from the sheet, creating bud-like shapes that lend organs their initial three-dimensional structures.
“The way you get the patterns of almost all your tissues is through progressive budding or branching in fractal-like ways. All of these patterns are dependent on branches on top of branches, or buds on top of buds,” Ingber says.
Squeezing out a tooth
Given the number of buds that grow on top of each other in a lung or a pancreas or a liver, breaking down how mechanical forces shape such complex structures is no easy task. “In the pancreas or in blood vessels, there are millions of branches and buds, in all three dimensions,” Ingber says. Instead, the best way to get a sense of how the process occurs is to distill it down to the formation of a single bud.
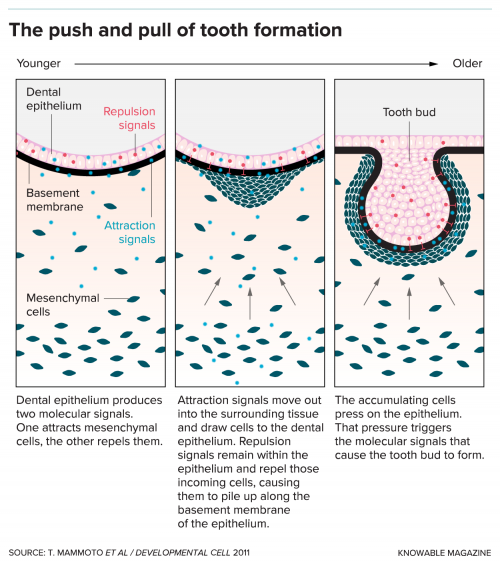
With that in mind, in an elegant 2011 study, he and his colleagues focused on one of the only organs that emerges naturally from a lone bud — a tooth. Rather than needing millions of branches to form its structure, it requires only one. That branch grows in two sections: a part that bows out to form the exterior, and another that pops inward to form roots. By investigating how and when those sections grew, Ingber’s team hoped to unravel the role of physical force in the process.
Researchers had known for years that proteins called motility factors play a role in this process. Usually, they beckon cells to grow toward them like diehard fans flocking toward a celebrity, which squeezes them together into a dense, taut mass. But what if the growth factors were just a means to an end? What if the key to tooth development wasn’t those proteins themselves, but the physical squeezing that they cause as cells crowd together?
To test this idea, Ingber and his colleagues first removed growth factors from the equation, and found that tooth development ground to a halt. Then they tried growing the developing buds between two sheets of a rubbery polymer. Shockingly, as they squeezed those sheets together, the tooth’s normal formation started once again. The scientists later showed that cells started to mineralize and lay down what looked like dentin, a key toothy building block. The force exerted on the cells by the polymer sheets alone was enough to kick off the entire cascade of development.
When force goes awry
The physical forces in and around early cells may spark the formation of organs — but they can also cause the body to fail in spectacular ways. An entire laundry list of seemingly unrelated diseases can be linked to errors in how cells sense force during and after development.
Spina bifida, a condition where an embryonic structure called the neural tube (which eventually forms the brain and spinal cord) fails to close, is connected to a breakdown in cellular forces, new research suggests. Likewise, cleft palate, which occurs when two sides of a person’s palate are deformed, could be caused by force-sensing gone awry at specific points during embryonic development.
Sometimes, the effects of these force-sensing glitches can lead to problems later in life, notes Lammerding. Some types of muscular dystrophy, a disease that results in muscle atrophy over time, may be linked to a breakdown in force-sensing mechanisms within muscle cells.
“Normally if you go to the gym to exercise, the muscles will adapt to the higher mechanical load,” Lammerding says. When muscle tissue feels the physical force of weight pulling on it, the cells inside it lay down more fibers of proteins called actin and myosin in their cytoskeletons. The more of those fibers the muscle cells contain, the stronger they get.
But in one form of the disease called Emery-Dreifuss muscular dystrophy, the cells have trouble sensing the forces stretching them, so they can’t create more muscle fiber in response to the load. As a result, they don’t grow stronger with exercise, and instead atrophy slowly on the bone. Although the symptoms of the disease set in several years after birth, their root cause is a breakdown in cellular force sensing, Lammerding says.
New treatments
For many of these diseases, there’s currently no way to prevent them before they emerge. But bioengineers are searching for new ways to harness the physical forces that shape cell development, and might one day be able to apply them in treatments.
The dream goal, Nelson says, would be to use mechanical force, along with other inputs, to grow tissues and organs on demand in the lab — a development that could do away with long waiting lists for transplants in patients with life-threatening diseases. “Ultimately, what I want to do is know this well enough that we can take a population of maybe a hundred cells, put them in a dish and provide them with two or three initiating stimuli, and have them build themselves into a lung,” she says. Granted, the idea is far-fetched, but based on how quickly research is progressing, she thinks we may see success in a decade or less.
In the meantime, other researchers are working on more immediate applications. Ingber, for one, is taking what he’s learning about the way cellular scaffolds and mechanical forces affect development to create lifelike organ tissues in the lab.
To accomplish that feat, he’s artificially steering the development of cells by growing them on surfaces that mimic the physical and chemical environment of specific organs. With the right mechanical stimulation, he says, cells placed there will develop into a fully formed layer of organ tissue. The result, called an organ-on-a-chip, provides a thin cross section of working kidney, liver or other organ, complete with its own three-dimensional structure.
Devices like these, he adds, could provide accurate models of full-sized organs within tiny pieces of translucent material, letting scientists and drug companies test new treatments for disease more rapidly and effectively than ever before.
Other researchers want to manipulate cellular forces to treat severe wounds. Treena Arinzeh, a biomedical engineer at the New Jersey Institute of Technology, is working to develop new types of materials that could help regrow large sections of missing bone, doing away with the need for metal implants or grafts from cadavers. By putting stem cells on a specially engineered scaffold — a sort of spongy structure made in the lab — she provides them with a bed that has just the right amount of stiffness to coax them into becoming bone and cartilage cells.
This, she hopes, can one day help treat injuries from trauma, weakened bones from osteoporosis, and potentially a whole host of other conditions. “Even though the fundamental science is still developing, we are trying to at least take some of that knowledge and develop therapies out of it,” she says. “We’re probably only right at the tip of the iceberg in terms of what we can do.”
As with any cutting-edge science, the knowledge that emerges from research like this will inform the next questions scientists ask to understand how the body takes shape. Eventually, cellular force-sensing might even become the basis for a radical new interpretation of developmental biology. The research is still in its early stages, and there are still many open mysteries about the ways mechanical forces steer the fate of cells. But the answers to those puzzles — and the new medical treatments that they enable — may surface within our lifetime.
Until then, I won’t need to look very far to revel in the unlikely miracle of the human form. Like all new parents, I’m struck each day by the profound wonder of a tiny person developing before my eyes. The growing confidence of little feet plodding across a bare wood floor; the wailing cries that arrive as new teeth sprout from sensitive gums; the tiniest milestones, new abilities and revelatory moments of a new body coming into its own — all of those made possible by the complex ballet of cells and tissue unfolding to create a small, sweet, curious little boy.
10.1146/knowable-012720-2
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews. Sign up for the newsletter.



 (No Ratings Yet)
(No Ratings Yet)