The home of the implanting embryo: A 3D perspective
Posted by Ripla Arora, on 5 January 2017
More than 2000 years ago, Hippocrates (460-377BC) and Aristotle (384-322BC) described the human uterus as a series of chambers with a lining of tentacles or suckers. They believed that blood vessels connected the breast to the uterus allowing the pumping of breast milk into the uterine cavity. In this way, the embryo would be nourished by suckling on milk through the uterine tentacles whilst simultaneously being prepared for breast-feeding after birth. These early anatomical descriptions were the product of animal studies and philosophical theory, as laws and religion prohibited dissection of cadavers. Much of our present day understanding of pregnancy and the nature of embryo-uterine interactions is founded upon the documentation of human anatomy by artists such as Johannes de Ketham (1470-1491) and Leonardo da Vinci (1452-1519) and photographic evidence due to advent of microscopy and histology in the 16th century.
Widespread use of anatomical drawings and photographs is proof that visualization is key to the understanding of mechanisms governing basic biology. Oddly enough, though the embryo itself is much smaller (and less visible) than the uterus, we have a deeper understanding of how the fertilized egg develops into the blastocyst stage embryo. This is the result of laboratory techniques in blastocyst isolation and embryo culture combined with live imaging of fluorescent labeled proteins in the transparent early embryo. Missing is an understanding of the interaction between the embryo and its niche, the uterine lining, and how the embryo is guided to its site of implantation for attachment and future growth.
While working in the laboratory of Diana Laird on a project involving 3D imaging of the intact ovary (Faire et al., 2015), she and I contemplated how our imaging method could be applied to studying other organs. Meanwhile, my studies of the non-canonical Wnt receptor Ror2 identified a high level of expression in the mouse and human endometrium (Arora et al., 2014), and I wondered about the spatial distribution of this expression. Putting the two together, we tested the ovarian method of whole mount immunofluorescence and confocal imaging on the intact uterus. This worked well for neonatal uteri (which are not as thick and don’t have much muscle), but poorly in adult uteri. I made some key changes to the way I fixed the uteri by preserving their in situ length and incubating with antibodies for multiple nights, which led to better penetration of the antibodies.
The confocal imaging for three different antigens in a full-length mouse uterus requires ~5 hours of imaging time (about 18X2 tiles at a 10X magnification) and generates a data file size of 10-12GB. Using Imaris, an image analysis software, I reconstructed 3D visualizations of non-pregnant and pregnant adult mouse uterine epithelium.
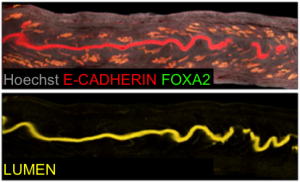
Our next goal was to isolate the uterine luminal epithelium independent of the glands. To this end, we computationally subtracted FOXA2+ glandular signal from the total E-CADHERIN+ epithelial signal. This gave rise to a lumen-only signal helping us generate the first 3D renderings of the mouse uterine lumen.
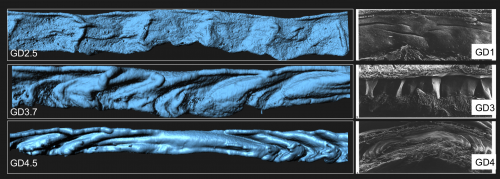
To our surprise, the 3D architecture of the uterine luminal epithelium was not static during preimplantation stages with many structural changes that would be impossible to discern using histology. Prior to implantation, the lumen undulated in stereotypical folds that were oriented perpendicular to the ovarian cervical axis. When overlapped with the optical Z-slices and compared with published 2D histological sections we determined that these folds are indeed structures known as uterine crypts.
We established a daily time course of the pattern of luminal folds during early pregnancy and ultimately their resolution around the site of implantation. Combing through literature, I also identified structural similarities between the luminal folding dynamics in my imaging and SEM images from the luminal side of rat uteri published in the 1990s (Winkelmann and Spornitz, 1997).
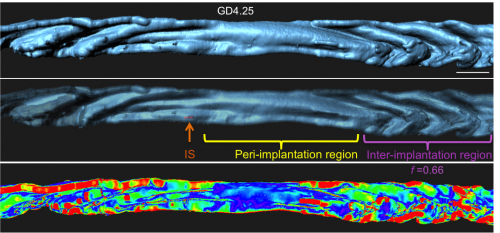
Next I faced the challenge of quantifying something that was quite obvious to the eye, but still needed measurement to confirm. We collaborated with the Biological Imaging Development Center at UCSF to work out a computational algorithm for quantifying the degree of luminal folding. With the surface renderings generated in Imaris, the algorithm calculated surface curvature and derived a simple expression for folding factor (f). The Matlab script for surface curvature interfaced with Imaris to depict the folds as a heat map where deep folds were painted red and flatter regions blue. This heat map helped us divide the entire uterine lumen into three segments – the implantation site (where the embryo is present), the peri-implantation region (flattened lumen) and the inter-implantation region (folded lumen).
We next used 3D imaging methods to elaborate on the function of uterine glands. Although ancient philosophers believed the uterus to be connected to the mammary gland, the breast and uterus were eventually shown to be anatomically distinct.
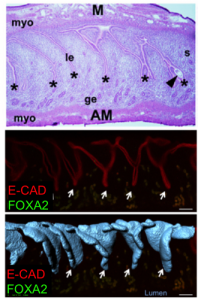
Additionally, the composition of the uterine milk was not composed of albumins, but rather, uterine secretions termed “the embryotrophe” secreted by the uterus’s own glands. In 2D histological sections, these glands appear as discrete epithelial structures floating in the stroma. We were keen to evaluate the clustering of glandular structures using previously described algorithms in the lab. Using FOXA2 as a marker for glands, our 3D imaging technique allowed us to show that that glands are actually branched structures connected to the uterine lumen by a small duct, giving the appearance of grapes on a vine. Surprisingly, the floating structures observed in 2D sections were connected to each other and to the uterine lining, when viewed in 3D. These glands are also present only at the antimesometrial side of the uterine lumen, where the embryo eventually implants in the pregnant uterus.
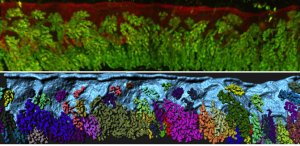
The ancient Greeks were right – uterine glands are still considered to be important for secreting substances that will nourish the embryo until the placenta is formed. Studies of farm animals and of mice have shown that glandular secretions are key to embryo development, as animals with defective glands are sub-fertile or infertile. However, no 3D imaging studies have been done to observe the organization of these glands or chart any changes in glands associated with implantation.
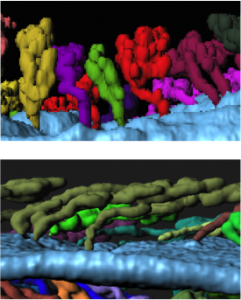
I noticed that the glands of the implantation stage uterus had a very characteristic organization. At this time, glandular ducts elongate and bend towards the site of implantation. This was a curious observation, considering that uterine glands are classically considered to be exocrine glands that secrete molecules into the uterine lumen (or down the trunk into the roots of the vine in my analogy). This ductal elongation would paradoxically increase the length of the path that glandular secretions have to travel before reaching the embryo. However, the glandular duct elongation also effectively brings the uterine glands in close spatial proximity to the stroma that will decidualize. These observations led us to hypothesize that uterine glands might secrete their factors into the stroma or into the closely associated vasculature and not just into the lumen, assisting in implantation and embryo growth.
All of this mouse work made us curious about the human uterine architecture. We were fortunate enough to obtain proliferative phase human endometrial hysterectomy samples through Dr. Linda Giudice in our department at UCSF. The glandular organization in humans is much more complex than in mice. Our method opens up possibilities to elaborate on the intricacies of epithelial organization in the human endometrium.
My vision is that this methodology will transform the way we view implantation. We will be able to elucidate the mechanisms by which implantation is disrupted in genetic mutants. Using these techniques, we are now beginning to ask questions about embryo spacing, crypt formation, and the interactions between the embryo and uterine epithelium as it travels through the lumen to find its site of attachment. This will lead to a better understanding of the conversation between the fetus and the maternal lining ultimately improving our approach towards – artificial reproductive technologies in the clinic, treatment of infertility and identifying novel targets for contraception.
References
Arora, R., Altman, E., Tran, N. D. And Laird, D. J. (2014). Novel domains of expression for orphan receptor tyrosine kinase Ror2 in the human and mouse reproductive system. Dev Dyn 243, 1037-1045.
Cha, J., Bartos, A., Park, C., Sun, X., Li, Y., Cha, S. W., Ajima, R., Ho, H. Y., Yamaguchi, T. P. and Dey, S. K. (2014). Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 8, 382-392.
Faire, M., Skillern, A., Arora, R., Nguyen, D. H., Wang, J., Chamberlain, C., German, M. S., Fung, J. C. and Laird, D. J. (2015). Follicle dynamics and global organization in the intact mouse ovary. Dev Biol 403, 69-79.
Teixeira, J., Rueda, B. R. and Pru, J. K. (2008) Uterine stem cells. In Stembook (ed: L. Girard), (Internet). Cambridge (MA): Harvard Stem Cell Institute.
Winkelmann, A. and Spornitz, U. M. (1997). Alkaline phosphatase distribution in rat endometrial epithelium during early pregnancy: a scanning electron-microscopic study. Acta Anat (Basel) 158, 237-246.


 (4 votes)
(4 votes)