If you give a scientist a cuttlefish . . .
Posted by Brent Foster, on 20 March 2023
She might not see it the next day. At first glance, the slag in the saltwater aquarium is just a rock covered with purple jasmine polyps and green star polyps. Orange tendrils of finger corals wave with a silent current, an undulating calm that almost seems to slow the heart rate. Suddenly the rock flutters. A part of it shifts, seems to detach and hover in the tank, and the shape of a dwarf cuttlefish emerges as bands of light and dark ripple across its skin.
“They’re like little artists creating impressionist paintings on their skin,” said Tessa Montague, a post-doc in the Axel lab at Columbia University. Her latest scientific mission is to understand how the cuttlefish brain controls dynamic camouflage behavior.
From the moment a cuttlefish is born, its skin already contains thousands of pigment-filled sacs called chromatophores that expand and contract under direct control of the brain. And, compared to more established model organisms like the mouse, the dwarf cuttlefish brain is huge. Nearly 75% of its brain is optic lobe, indicating just how important vision is for cuttlefish to sense the world around them.
“When a cuttlefish sees an object or visual scene,” Tessa explained, “the brain must create an abstract representation of the scene using patterns of neural activity.” But through the secret language of neurons the cuttlefish undergoes a remarkable transformation and recreates an approximation of what it has seen on its skin through the fine control of its chromatophores. By studying this transformation, Tessa and colleagues have an opportunity to learn fundamental principles about how brains process the visual world.
Studying a brain as alien as the cuttlefish requires tools to manipulate the genome and record neuronal activity. The way to to do that is to utilize the cuttlefish’s development to create a transgenic animal. But it’s challenging to design experiments without knowing what’s possible or most likely to succeed. At the start of Tessa’s project, no one had sequenced the dwarf cuttlefish genome, and no one had adapted any of the myriad transgenic tools for cephalopods. How hard could it be?

Tessa and colleagues from UCSD and the Chan-Zuckerberg Initiative sequenced the dwarf cuttlefish genome — a 5.5 GB string of DNA, almost twice the size of the human genome. Then, with colleagues at the Marine Biological Laboratory in Woods Hole, Massachusetts, they sequenced RNA transcripts and assembled the cuttlefish transcriptome. Unfortunately, these were not sufficient to identify candidate transgenic drivers. Unfazed, they teamed up with a group at Columbia to perform ATAC-seq — a method that identifies regions of open chromatin in the genome, revealing hidden stretches of non-coding DNA near a gene that determines when and where that gene will be expressed. Scientists can hijack these promoter regions and repurpose them to drive a transgene of their choosing, usually something that glows. Find the right promoter to drive the right gene, and voila! You have a transgenic construct that will be expressed in a specific population of cells. Tessa’s goal is to drive GCaMP, a fluorescent indicator of neural activity, in the cuttlefish brain.
“After many trials and tribulations, we found a really good promoter within the actin gene,” Tessa told scientists at the Society for Integrative and Comparative Biology (SICB) 2023 Conference in January. “We also identified some promising neuronal promoters, which is very exciting.” But getting a promoter to transiently drive expression of a transgene isn’t good enough. To establish a stable transgenic line, the transgene needs to be integrated directly into the genome so it can be passed on to the offspring. And that requires the right tools.
There’s no shortage of possibilities. For decades, scientists have been fiddling with different transgenic tools, from transposases to Zinc-fingers to restriction enzymes to CRISPR-Cas9.
Tessa’s research team has tried several of these tools, with limited success. Most recently, while teaching the embryology course at the Marine Biological Laboratory, a student told her about a special protocol using the meganuclease I-SceI that creates transgenic frogs and fish very efficiently. Tessa’s testing it now in her dwarf cuttlefish.
“Every time I describe the method I’m using, someone says, ‘Have you thought about this?’ and they offer me a different approach,” said Tessa. “The community support has been amazing, but I try to avoid the temptation to constantly jump between methods because each one probably requires months of adaptations for the cuttlefish. And I won’t know for sure if any method has worked until months later.” It can be difficult balancing the decision of when to spend time and resources on giving something a fair chance versus when to move on.
Injecting cuttlefish embryos offers its own host of challenges, not least of which is getting the cuttlefish parents to cooperate in the first place. During mating, the male cuttlefish embraces the female with his eight arms and deposits his spermatophores into her cheeks. The female can then store the sperm for weeks, and she decides when to fertilize and lay her eggs at her own leisure.
“They tend to want to do this on weekends and in the middle of the night, which is not very good for us,” Tessa told scientists at SICB. She came up with a simple trick of putting a box with large openings inside the tank. The cuttlefish generally don’t like the box because it’s too exposed. But place a rock on top and it suddenly becomes a safe haven. “Almost like clockwork, within three hours the cuttlefish swim inside and lay a bunch of eggs,” said Tessa.
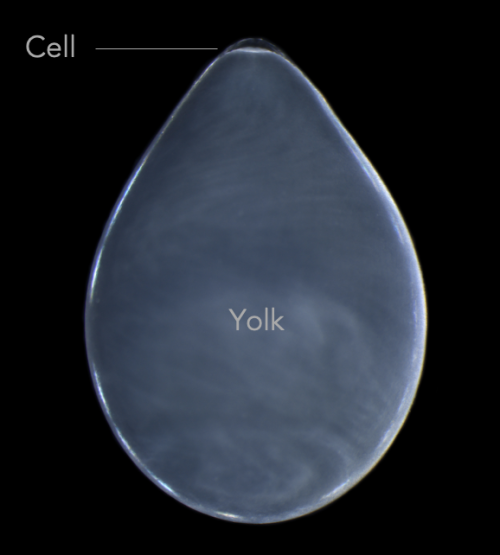
The eggs themselves are covered in dozens of layers of inky jelly that need to be removed before injecting. If you just tear away the jelly and try to grow the embryos in a dish, they die. The solution? Dunk the embryos in bleach and then gently rinse them off. After this bleach treatment, the ink jelly sloughs off easily and researchers can plop the embryo into a dish with antibiotics and squash it between two plastic tubes. This creates a positive pressure that, when combined with a quartz needle beveled at 15º to a very sharp 2.5-micron tip, allow researchers to punch through a tough chorion to reach the teensy cell at the top of a glob of yolk.

Left: The so-called “Holy Temple”: an open box with holes and a rock on top with an inky clutch of cuttlefish eggs. Right: Closeup image of a de-jellied cuttlefish egg. (Photo credits: Tessa Montague)
“No matter how delicately we do this, the vast majority of injected embryos die,” said Tessa, “but after years of practice, enough survive that we can do some experiments.” It takes four months, filled with lots of food and care, before the cuttlefish become sexually mature and Tessa can see if her transgenes make it to the germ line and are passed on to another generation of little cuttlefish.
Even without the worry of death by injection, it takes quite a lot of effort to keep cuttlefish alive. Two full-time animal specialists count grass and mysid shrimp and offer them one by one to the 200 dwarf cuttlefish housed in the Axel lab at Columbia University. Because of their fast metabolism, cuttlefish must be fed three times a day, resulting in lots and lots of poop. And they’re extremely sensitive to changes in water quality, meaning the animal tanks must feature myriad filtration systems and be cleaned every day.
“I’m very fortunate to have a team of six or seven people that work with me,” said Tessa. “Creating something out of nothing is very difficult. But every day that goes by, things get a little easier.”
Tessa wants to share her research to inspire other scientists whose burning scientific questions in new model organisms also require technological developments to answer them. To that end, she created Cuttlebase, a website that chronicles the scientific tools her team has developed, including a 3D brain atlas and a staging series of dwarf cuttlefish embryonic development. Her outreach extends beyond scientists to the general public — her personal website hosts a CuttleCam livestream where anyone in the world can watch these alien creatures from the comfort of their own home.
So, if you give a scientist a cuttlefish, know that she may embark on a journey to understand its secret language of colors rippling across its skin. It may be bumpy, but that journey will have at least a few moments that will take her breath away. And if you know where to look — if you can find the fluttering fins of a cuttlefish hidden in the rock — you might find yourself holding your own breath on your own journey. Maybe that just confirms there’s a scientist in all of us.


 (3 votes)
(3 votes)