In Development this week (Vol. 137, Issue 21)
Posted by Seema Grewal, on 12 October 2010
Here are the research highlights from the current issue of Development:
Oct1: essential for trophoblast development
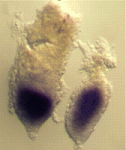 Most POU family transcription factors are temporally and spatially restricted during development and play pivotal roles in specific cell fate determination events. Oct1 (Pou2f1), however, is ubiquitously expressed in embryonic and adult mouse tissues; so, does Oct1 have a developmental role? On p. 3551, Fatima Cavaleri and colleagues report that Oct1 regulates trophoblast development during mouse embryogenesis. The researchers generate Oct1-null embryos and show that they fail to develop beyond the early primitive streak stage. Analysis of the mutant embryos reveals that they lack normal maternal-embryonic interfaces because of reduced extra-embryonic ectoderm (ExE) formation and the absence of the ectoplacental cone – two extra-embryonic tissues generated from the trophectoderm cells that overlie the inner cell mass (which forms the embryo). Other experiments indicate that Oct1 loss is incompatible with the derivation of trophoblast stem cells, which normally reside in the ExE. The researchers suggest, therefore, that Oct1 is primarily required for the maintenance and differentiation of the trophoblast stem cell compartment during early post-implantation development.
Most POU family transcription factors are temporally and spatially restricted during development and play pivotal roles in specific cell fate determination events. Oct1 (Pou2f1), however, is ubiquitously expressed in embryonic and adult mouse tissues; so, does Oct1 have a developmental role? On p. 3551, Fatima Cavaleri and colleagues report that Oct1 regulates trophoblast development during mouse embryogenesis. The researchers generate Oct1-null embryos and show that they fail to develop beyond the early primitive streak stage. Analysis of the mutant embryos reveals that they lack normal maternal-embryonic interfaces because of reduced extra-embryonic ectoderm (ExE) formation and the absence of the ectoplacental cone – two extra-embryonic tissues generated from the trophectoderm cells that overlie the inner cell mass (which forms the embryo). Other experiments indicate that Oct1 loss is incompatible with the derivation of trophoblast stem cells, which normally reside in the ExE. The researchers suggest, therefore, that Oct1 is primarily required for the maintenance and differentiation of the trophoblast stem cell compartment during early post-implantation development.
Schwann cells: more than just insulators
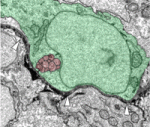 How neurons connect to their targets during embryogenesis has been intensively studied, but what maintains the position and connections of nerves during postembryonic growth? To investigate this, William Talbot and colleagues study the development of the posterior lateral line nerve (PLLn) in zebrafish embryos and larvae (see p. 3643). Using transmission electron microscopy, the researchers show that the PLLn – a peripheral nerve that innervates sensory organs in the epidermis – initially grows in the epidermis but that shortly after axon outgrowth, the epidermal basement membrane degrades and reforms on the nerve’s opposite side, thereby repositioning the nerve into the subepidermal space. Analysis of mutant and chimeric embryos shows that Schwann cells, which myelinate peripheral nervous system axons, are required for this process; without them, the PLLn becomes severely disorganised. Thus, by remodelling tissues in the vicinity of nerves, Schwann cells, which are traditionally regarded as static insulators, could play an important role in the proper organisation of nerves that innnervate other sensory organs during postembryonic growth.
How neurons connect to their targets during embryogenesis has been intensively studied, but what maintains the position and connections of nerves during postembryonic growth? To investigate this, William Talbot and colleagues study the development of the posterior lateral line nerve (PLLn) in zebrafish embryos and larvae (see p. 3643). Using transmission electron microscopy, the researchers show that the PLLn – a peripheral nerve that innervates sensory organs in the epidermis – initially grows in the epidermis but that shortly after axon outgrowth, the epidermal basement membrane degrades and reforms on the nerve’s opposite side, thereby repositioning the nerve into the subepidermal space. Analysis of mutant and chimeric embryos shows that Schwann cells, which myelinate peripheral nervous system axons, are required for this process; without them, the PLLn becomes severely disorganised. Thus, by remodelling tissues in the vicinity of nerves, Schwann cells, which are traditionally regarded as static insulators, could play an important role in the proper organisation of nerves that innnervate other sensory organs during postembryonic growth.
WUSCHEL robustly plants stem cell homeostasis
 Plant stem cell populations are maintained by the precise coordination of stem cell division and the rates of cell division and differentiation among stem cell progenitors. In the growing tips of higher plants (shoot apical meristems, SAMs), stem cell daughters produced by infrequent stem cell division in the central zone (CZ) are displaced towards the surrounding peripheral zone (PZ), where they divide faster and their progeny differentiate into leaves or flowers. Now, Venugopala Reddy and co-workers report that the homeodomain transcription factor WUSCHEL (WUS) mediates stem cell homeostasis in Arabidopsis (see p. 3581). The researchers use transient manipulation of WUS expression and live imaging to show that elevated WUS levels in the CZ induce CZ expansion and increase PZ cell division rates. Conversely, decreased WUS levels lead to a smaller CZ, reduced PZ cell division rates and increased responsiveness of PZ cells to the plant hormone auxin, which leads to enlarged organ primordia. Thus, by regulating stem cell numbers and growth and differentiation patterns, a single transcription factor robustly mediates plant stem cell homeostasis.
Plant stem cell populations are maintained by the precise coordination of stem cell division and the rates of cell division and differentiation among stem cell progenitors. In the growing tips of higher plants (shoot apical meristems, SAMs), stem cell daughters produced by infrequent stem cell division in the central zone (CZ) are displaced towards the surrounding peripheral zone (PZ), where they divide faster and their progeny differentiate into leaves or flowers. Now, Venugopala Reddy and co-workers report that the homeodomain transcription factor WUSCHEL (WUS) mediates stem cell homeostasis in Arabidopsis (see p. 3581). The researchers use transient manipulation of WUS expression and live imaging to show that elevated WUS levels in the CZ induce CZ expansion and increase PZ cell division rates. Conversely, decreased WUS levels lead to a smaller CZ, reduced PZ cell division rates and increased responsiveness of PZ cells to the plant hormone auxin, which leads to enlarged organ primordia. Thus, by regulating stem cell numbers and growth and differentiation patterns, a single transcription factor robustly mediates plant stem cell homeostasis.
Haematopoietic cluster locations made transparent
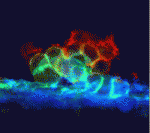 Haematopoietic clusters – cell aggregates that are associated with endothelium in the large blood vessels of midgestation vertebrate embryos – play a pivotal but poorly understood role in the formation of the adult blood system. To date, the opaqueness of whole embryos has prevented the systematic quantitation or mapping of all the haematopoietic clusters in mouse embryos but, on p. 3651, Tomomasa Yokomizo and Elaine Dzierzak remedy this situation. Using a technique to make whole mouse embryos transparent, combined with immunostaining and three-dimensional confocal microscopy, they show that the number of clusters peaks at embryonic day 10.5. Clusters are heterogeneous, they report, and localise to specific vascular subregions, such as the middle subregion of the aorta near to its junction with the vitelline artery. Finally, by combining flow cytometry and functional studies, the authors demonstrate that haematopoietic progenitor and stem cells are enriched within the cluster population. Together, these results provide novel insights into the spatial development of the adult blood system in mice.
Haematopoietic clusters – cell aggregates that are associated with endothelium in the large blood vessels of midgestation vertebrate embryos – play a pivotal but poorly understood role in the formation of the adult blood system. To date, the opaqueness of whole embryos has prevented the systematic quantitation or mapping of all the haematopoietic clusters in mouse embryos but, on p. 3651, Tomomasa Yokomizo and Elaine Dzierzak remedy this situation. Using a technique to make whole mouse embryos transparent, combined with immunostaining and three-dimensional confocal microscopy, they show that the number of clusters peaks at embryonic day 10.5. Clusters are heterogeneous, they report, and localise to specific vascular subregions, such as the middle subregion of the aorta near to its junction with the vitelline artery. Finally, by combining flow cytometry and functional studies, the authors demonstrate that haematopoietic progenitor and stem cells are enriched within the cluster population. Together, these results provide novel insights into the spatial development of the adult blood system in mice.
Chordin downregulation waves on aortae fusion
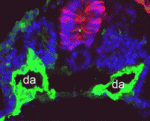 During development, extensive remodelling of the embryonic vasculature, the first organ to develop, ensures that the embryo’s cells are constantly supplied with oxygen and nutrients. The first major vascular remodelling event in mammalian and avian embryos is fusion of the bilateral dorsal aortae at the midline to form the dorsal aorta. Takashi Mikawa and co-workers now show that a developmental switch in notochord activity signals this fusion in chick and quail embryos (see p. 3697). Prior to fusion, the researchers report, the notochord secretes positive and negative factors that together block the initiation of aortae fusion. Notably, whereas the expression of positive vascular regulators is maintained during fusion, the expression of negative regulators such as chordin, an antagonist of the positive regulator BMP, is downregulated along the anteroposterior axis. The discovery of this novel mechanism for modifying vascular pattern – modulation of vascular inhibitors without changes in positive vascular regulator levels – could aid the development of treatments for vascular injury.
During development, extensive remodelling of the embryonic vasculature, the first organ to develop, ensures that the embryo’s cells are constantly supplied with oxygen and nutrients. The first major vascular remodelling event in mammalian and avian embryos is fusion of the bilateral dorsal aortae at the midline to form the dorsal aorta. Takashi Mikawa and co-workers now show that a developmental switch in notochord activity signals this fusion in chick and quail embryos (see p. 3697). Prior to fusion, the researchers report, the notochord secretes positive and negative factors that together block the initiation of aortae fusion. Notably, whereas the expression of positive vascular regulators is maintained during fusion, the expression of negative regulators such as chordin, an antagonist of the positive regulator BMP, is downregulated along the anteroposterior axis. The discovery of this novel mechanism for modifying vascular pattern – modulation of vascular inhibitors without changes in positive vascular regulator levels – could aid the development of treatments for vascular injury.
Cut to fly airway remodelling
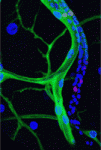 In insects that completely metamorphose, such as Drosophila, embryonically specified imaginal cells remain dormant until the larval stages when their coordinated proliferation and differentiation generates various adult organs. Now, on p. 3615, Chrysoula Pitsouli and Norbert Perrimon describe how embryonic cells – spiracular branch (SB) tracheoblasts – remodel the Drosophila abdominal airways during metamorphosis. The adult fly tracheal system consists of branched tracheal tubes (which transport air into the insect’s body) and spiracles (the external respiratory organs, which are surrounded by epidermal cells). The researchers show that embryonic SB tracheoblasts are multipotent cells that express the homeobox transcription factor Cut, which is necessary for their survival and normal development. SB tracheoblasts, they report, give rise to three distinct cell populations at the end of larval development, which generate the two components of the adult tracheal system and the surrounding epidermis. This dissection of the molecular events that underlie the formation of an adult tubular structure in Drosophila might shed light on mammalian tubular organ development, suggest the researchers.
In insects that completely metamorphose, such as Drosophila, embryonically specified imaginal cells remain dormant until the larval stages when their coordinated proliferation and differentiation generates various adult organs. Now, on p. 3615, Chrysoula Pitsouli and Norbert Perrimon describe how embryonic cells – spiracular branch (SB) tracheoblasts – remodel the Drosophila abdominal airways during metamorphosis. The adult fly tracheal system consists of branched tracheal tubes (which transport air into the insect’s body) and spiracles (the external respiratory organs, which are surrounded by epidermal cells). The researchers show that embryonic SB tracheoblasts are multipotent cells that express the homeobox transcription factor Cut, which is necessary for their survival and normal development. SB tracheoblasts, they report, give rise to three distinct cell populations at the end of larval development, which generate the two components of the adult tracheal system and the surrounding epidermis. This dissection of the molecular events that underlie the formation of an adult tubular structure in Drosophila might shed light on mammalian tubular organ development, suggest the researchers.
Also…

In the first of our new Evolutionary crossroads in developmental biology series, Prigge and Bezanilla introduce Physcomitrella patens, a moss from this ancient, non-vascular plant lineage, studies of which are distinguishing ancestral developmental mechanisms from those that are novel innovations in flowering plants. See the Primer on page 3535.
This article is the first in a series of Primers on organisms and phyla that have been particularly informative for studying the evolution of developmental mechanisms and morphology. Other articles in this series will be published over the next 12 months.


 (No Ratings Yet)
(No Ratings Yet)