In Development this week (Vol. 137, Issue 22)
Posted by Seema Grewal, on 26 October 2010
Research highlights from the current issue of Development:
Novel Hh targets fly in
Hedgehog (Hh), a secreted morphogen, acts in a paracrine fashion to regulate tissue patterning during embryogenesis. Its tissue-specific effects are mediated by the transcription factor Cubitus interruptus (Ci), but how it exerts such effects is unclear. On p. 3887, Thomas Kornberg and colleagues address this question by identifying novel Drosophila Hh targets. Using chromatin-binding experiments to identify genes that are bound by Ci during Drosophila organogenesis, and by using expression data from wild-type embryos and Hh pathway mutants, they identified a set of Hhresponsive genes, many of which represent novel targets. Their validation of these targets in developing tissues, such as the dorsal ectoderm, showed that they are expressed in a tissue-specific manner, but, unexpectedly, that some targets are induced in an autocrine fashion. The authors also show that, in the tracheal primordium, some Hh target expression is subject to combinatorial control by Ci and an Hh-independent transcription factor. These unexpected features of Hh signalling provide new insights into our understanding of this pathway.
Modelling to get a head(fold)
Formation of the head fold (HF), the first three-dimensional structure to form in the embryo, is a crucial event that initiates heart, foregut and brain development. Although the molecular factors involved in HF development are becoming known, the biophysical mechanisms governing this transformation have yet to be investigated. Now, on p. 3801, Larry Taber and colleagues combine experimental and modelling approaches to determine the forces that drive HF formation in chick embryos. They generated a computational model for HF formation, and by inducing three distinct morphogenetic mechanisms – convergent extension in the neural plate (NP), cell wedging along the anterior NP border, and cell shaping outside the NP – they were able to simulate HF formation in this model. By comparing the changes in tissue morphology, mechanical strains and regional tissue stresses observed in the model with those measured experimentally in ex ovo embryos, the authors confirm that these three modelled morphogenetic mechanisms can alone provide the forces that drive HF formation.
Embryonic variations on a histone theme
Numerous histone variants exist in eukaryotes, and the replacement of canonical histones with such variants probably contributes to chromatin remodelling. Chromatin remodelling occurs during fertilisation, as germ cells become totipotent zygotes, but the role of histone variants during this process is unknown. On p. 3785, Fugaku Aoki and colleagues assess the dynamics of histone H2A and its variants, H2A.X, H2A.Z and macroH2A, during mouse oogenesis and pre-implantation development. They report that all variants are present in oocytes; by contrast, only H2A.X is abundant in one-cell embryos. The authors confirmed this postfertilisation reduction in H2A, H2A.Z and macroH2A using transgenic mice that express tagged H2 variants, and by microinjecting embryos with mRNA for these variants. Domain-swapping experiments showed that the C-terminal 23 amino acids of H2A.X enable its incorporation into chromatin after fertilisation, and that the concomitant reduction of H2A.Z and macroH2A is required for normal development. The authors suggest that altered histone composition might therefore contribute to the genome remodelling, and hence reprogramming, that occurs postfertilisation.
Meristem homeostasis: it takes three

In plants, the shoot apical meristem (SAM) provides all the cells that are needed for post-embryonic growth and development of the leaves, stems and flowers. In Arabidopsis, a peptide ligand derived from CLAVATA3 (CLV3) regulates the SAM stem cell pool by signalling through two receptor complexes – a homodimer of the receptor-like kinase CLV1 and a heterodimer consisting of the receptor-like protein CLV2 and the protein kinase CRN/SOL2. Now, Shinichiro Sawa and colleagues report that the receptor-like kinase RPK2 also has a vital role in SAM maintenance (see p. 3911). They show that loss-of-function mutations in RPK2 result in SAM stem cell expansion and increased numbers of floral organs, as seen in clv1 and clv2 mutants. Notably, the RPK2 mutant phenotypes are additive with those of clv1 and clv2 mutations. Moreover, biochemical analyses in Nicotiana benthamiana reveal that RPK2 forms homodimers but does not associate with CLV1 or CLV2. The researchers propose, therefore, that three, rather than two, CLV3 signalling pathways regulate meristem homeostasis.
Lymphangiogenesis: macrophages show restraint
Lymphatic vessels play crucial roles during embryogenesis and in adult animals but the origin of their progenitor cells is controversial. Recent studies have suggested that during neo-lymphangiogenesis in inflammatory settings, macrophages transdifferentiate into lymphatic endothelial cells and/or release prolymphangiogenic growth factors, but are these mechanisms involved in the development of the normal lymphatic vasculature? On p. 3899, Natasha Harvey and co-workers suggest that the answer to this question is no. Using lineage tracing, the researchers show that lymphatic endothelial cells arise independently of the myeloid lineage during both embryogenesis and tumourstimulated lymphangiogenesis in the mouse. Thus, they suggest, macrophages are not the source of lymphatic endothelial progenitor cells in these settings. Furthermore, the dermal lymphatic vasculature in macrophage-deficient mice is hyperplastic because of increased lymphatic endothelial cell proliferation. So, rather than providing pro-lymphangiogenic growth factors, macrophages provide signals that restrain lymphatic endothelial cell proliferation. Given these results, any attempt to treat disease-stimulated lymphangiogenesis by targeting macrophages needs careful consideration, conclude the researchers.
Grainyhead heads up apical junction formation
Epithelial cell differentiation requires the formation of the apical junctional complex, a membrane-associated structure that includes adherens junctions (which mediate stable adhesion between epithelial cells) and tight junctions (which regulate the movement of water and solutes between epithelial cells). Now, on p. 3835, Kai Schmidt-Ott and colleagues report that the mammalian transcription factor grainyhead-like 2 (Grhl2), an epithelium-specific homologue of Drosophila Grainyhead, regulates the molecular composition of the apical junctional complex. Grhl2, they report, determines the expression levels of E-cadherin and claudin 4 (Cldn4) – key components of adherens junctions and tight junctions, respectively – in several types of epithelia. Other experiments reveal that Grhl2 regulates epithelial differentiation in vitro and in vivo, that Grhl2 deficiency in mice results in defective neural tube closure, and that Grhl2 associates with conserved cisregulatory elements in the Cldn4 and E-cadherin genes. Together, these data suggest that Grhl2 is a transcriptional activator of apical junctional complex components and is, therefore, a crucial participant in epithelial differentiation.
Also…
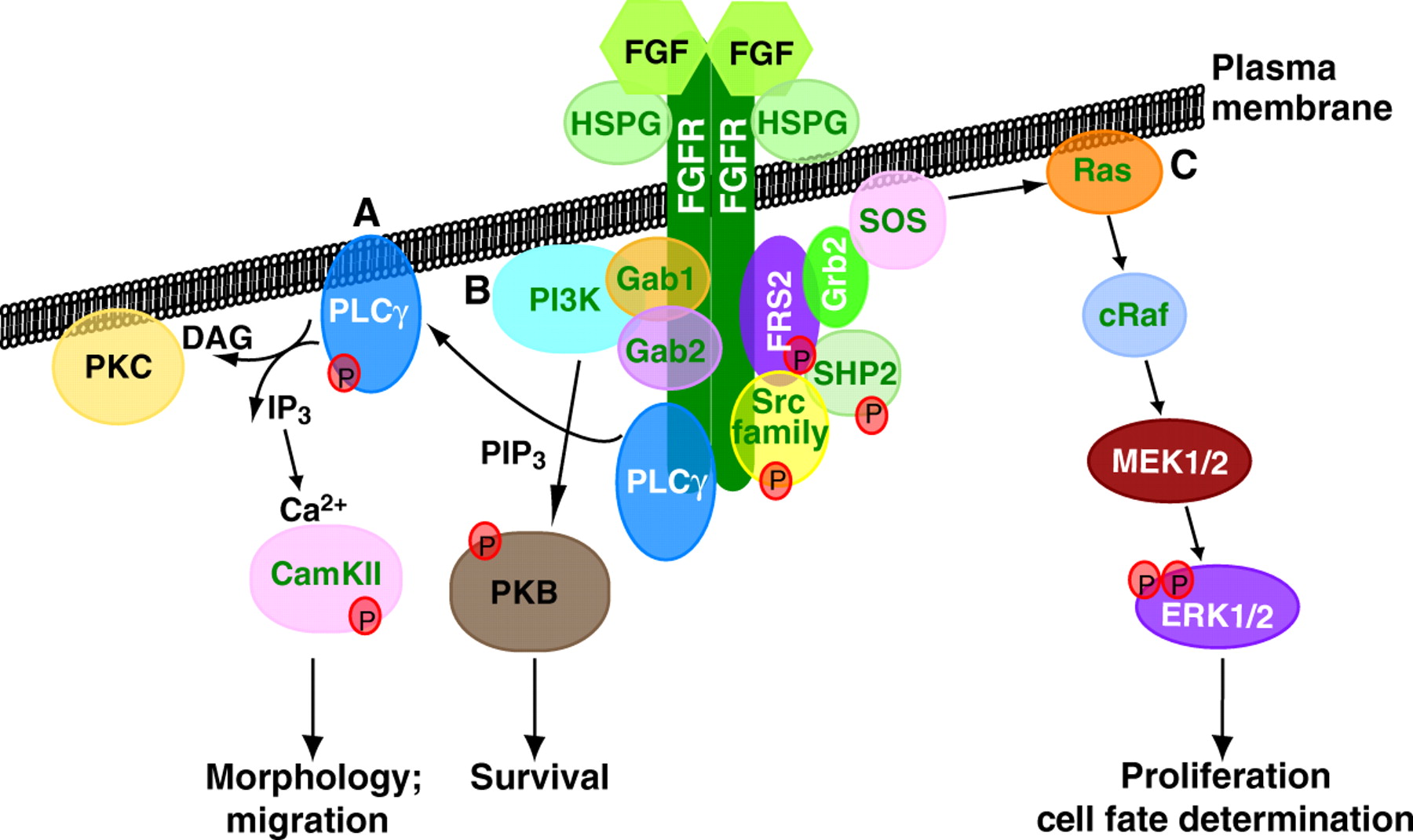
Since its discovery, FGF signalling has been implicated in numerous developmental processes and in disease. Now Karel Dorey and Enrique Amaya provide an update of the main developmental processes for which FGF signalling is vital during early vertebrate embryogenesis.
For more details, see the Primer article on p. 3731.


 (No Ratings Yet)
(No Ratings Yet)