In Development this week (Vol. 137, Issue 23)
Posted by Seema Grewal, on 9 November 2010
The current issue of Development is now online! Here are the research highlights:
Klf5: a multifaceted regulator of cell fate
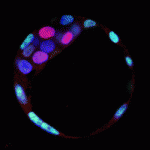
Kruppel-like transcription factors (Klfs) induce and maintain pluripotency in embryonic stem cells (ESCs), and Klf4 is one of the factors used to create reprogrammed iPS cells. The role of Klfs in the specification of the three lineages of the pre-implantation embryo – trophectoderm (TE), epiblast (EPI) and primitive endoderm (PE) – however, is not known. On p. 3953, James Wells and colleagues report that Klf5 is a dynamic regulator of all three lineages. Using Klf5 mutant mice, they show that Klf5 deficiency results in developmental arrest at the blastocyst stage, and causes defective TE formation, reduced EPI marker expression and increased PE marker expression in blastocysts. Conversely, overexpression of Klf5 suppresses the PE lineage in blastocysts and upregulates pluripotency-related genes in ESCs. Finally, Klf5-deficient blastocysts in culture fail to form pluripotent colonies and instead have an increased contribution of PE cells compared with control embryos. The authors conclude that Klf5 is a multifaceted regulator of cell fate specification during pre-implantation development.
LGL-1 on PAR with polarity
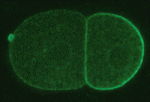
In the early C. elegans embryo, polarity is established via myosin-dependent contractions that lead to the asymmetric distribution of partitioning-defective (PAR) proteins; PAR-3 and PAR-6, together with the atypical protein kinase C (PKC-3), localize to the anterior cortex, whereas PAR-2 becomes enriched at the posterior cortex. In Drosophila and mammals, PAR-2 is not expressed, but numerous proteins, including Lethal giant larvae (Lgl), act together with the other PAR proteins to establish polarity. Kenneth Kemphues and colleagues (p. 3995) show that the C. elegans homolog of Lgl, LGL-1, functions redundantly with PAR-2 to maintain polarity in the C. elegans embryo. Like PAR-2, LGL-1 localizes to the posterior cortex of the embryo in a PKC-3-dependent manner, and its overexpression is sufficient to rescue loss of PAR-2 function. Importantly, they show that LGL-1 prevents myosin from accumulating in the posterior cortex of the embryo. This provides new insights into the way in which LGL-1 might influence myosin-dependent contractile flows and PAR protein localization, and hence cell polarity.
Nervous asymmetry

Left-right asymmetry is a conserved, but poorly understood, feature of animal nervous systems. Now, Robert Horvitz and colleagues reveal how a neuronal bilateral asymmetry is established in C. elegans (p. 4017). In C. elegans, the left-right asymmetric ABaraap cell lineage generates the single unpaired MI neuron and the e3D epithelial cell on the right and left sides, respectively, of the animal. The researchers show that the proneural bHLH genes ngn-1 and hlh-2, and the Otx homeodomain gene ceh-36 specify the MI neuron and establish this asymmetry – the determination of which occurs in the precursor cells for the left and right branches of the ABaraap lineage. Importantly, this initially cryptic asymmetry triggers activation on the right side only of a transcriptional cascade that then acts through multiple rounds of cell division, with CEH-36 functioning in the MIgrandmother cell, but not in the e3D-grandmother cell, to induce expression of NGH-1/HLH-2 in the MI-mother cell. Given their results, the researchers suggest that an evolutionarily conserved Otx/bHLH pathway establishes nervous system bilateral asymmetry in C. elegans and in other animals.
Shh: homeodomain interpreters at work
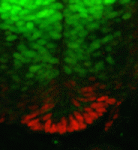
Morphogen gradients play an important role in establishing cell diversity during development. But how are small differences in the concentration of extracellular signals translated into a precise, robust transcriptional output in responding cells? On p. 4051, Johan Ericson and colleagues reveal that a homeodomain transcription factor feedback circuit is involved in the interpretation of the Sonic hedgehog (Shh) gradient that patterns the vertebrate ventral neural tube. They report that Nkx2 homeodomain proteins, which are induced by Shh, amplify Shh responses and are required for the induction of floor plate (FP) cells and p3 progenitors, the ventral-most neural tube cells. By contrast, the Pax6 homeodomain protein suppresses ventral fates by antagonising Shh signalling. Finally, the researchers report that a temporal switch in neural potential, rather than exposure of cells to different Shh concentrations, determines the spatial patterning of FP cells and p3 progenitors. They conclude, therefore, that dynamic, non-graded changes in responding cells are essential for the interpretation of graded Shh signalling.
Vascular instruction of liver development
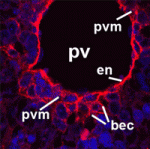
Alagille syndrome (AGS), which is caused by mutations in the Notch ligand jagged 1 (JAG1), is characterized by defective intrahepatic bile duct (IHBD) formation, but the mechanistic origins of this defect have been unclear. Now, on p. 4061, Luisa Iruela-Arispe and colleagues report that the conditional inactivation of Jag1 specifically in the developing portal vein mesenchyme (PVM), and not in the PV endothelium, of mice gives rise to AGS-like liver defects. They demonstrate that loss of Jag1 from the PVM leads to defective IHBD morphogenesis. Cytokeratin-positive bilary epithelial cells (BECs) surround the portal vein of these mice, indicating that their initial specification is Jag1 independent, but these cells fail to develop into mature bile ducts. Using in vitro spheroid co-cultures of isolated BECs and PVM, the authors show that loss of Jag1 from the PVM inhibits BEC aggregation, demonstrating that the PVM is a vital source of Jag1 signalling during BEC morphogenesis. The authors, thus, propose that the developing vasculature provides instructive signals for liver morphogenesis.
War of the whorls
Animal bristles, hairs and other surface appendages are orientated with the body axes and with adjacent structures to form precise macroscopic patterns. Unusually, in frizzled 6-null (Frz6–/–) mice, the hair follicles are orientated randomly in utero but reorientate after birth to create large-scale hair patterns. Jeremy Nathans and coworkers now describe the spatial and temporal dynamics of this hair follicle reorientation process (p. 4091). By analysing follicle orientations in Fz6–/– mice during late gestation and early postnatal life, they discover that an apparently local alignment pattern generates macroscopic patterns that compete with each other. Reorientation of the hair follicles at the junctions between different territories leads to the formation of whorls and crosses, which disappear within a week as the territories expand to generate long-range order. The researchers suggest that mouse hair follicle reorientation, which closely resembles the wing and thoracic hair realignments seen in Drosophila planar cell polarity mutants, could be driven by a follicle repulsion or interfollicle chemoattractant mechanism.
Also…
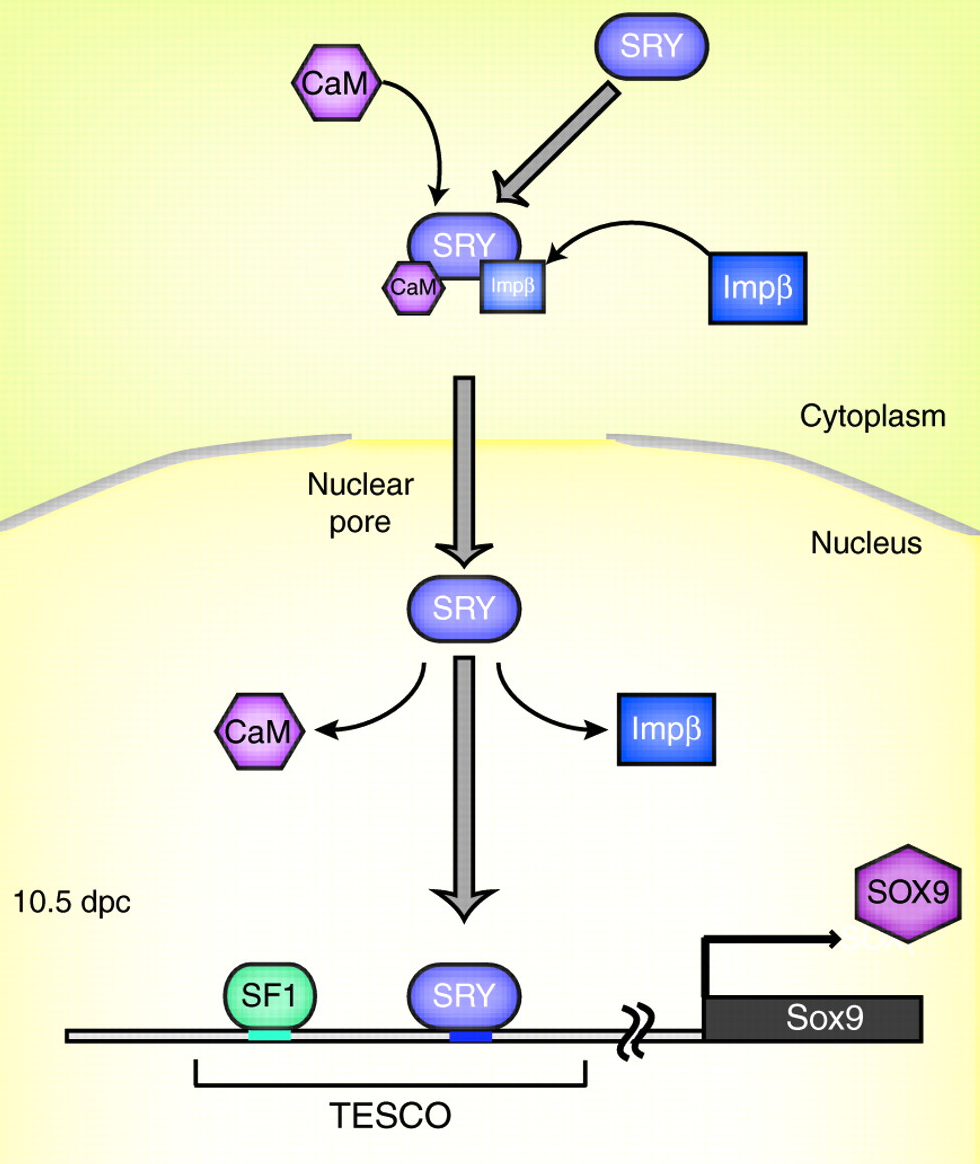 SRY is the transcription factor product of the sex-determining gene on the mammalian Y chromosome. In this issue, Kashimada and Koopman provide an updated account of how SRY triggers the cascade of molecular events that drive testis formation while inhibiting ovarian development.
SRY is the transcription factor product of the sex-determining gene on the mammalian Y chromosome. In this issue, Kashimada and Koopman provide an updated account of how SRY triggers the cascade of molecular events that drive testis formation while inhibiting ovarian development.
See the Primer article on p. 3921


 (No Ratings Yet)
(No Ratings Yet)