In Development This Week (Vol. 138, Issue 22)
Posted by Seema Grewal, on 25 October 2011
Here are the highlights from the current issue of Development:
The skin-healing touch of Lhx2
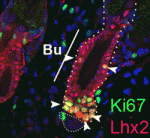
Skin repair after injury involves the recruitment of undifferentiated progenitor cells from nearby hair follicles (HFs) into the regenerating epidermis. The bulge and the secondary hair germ of HFs contain distinct populations of epithelial stem cells, and now Vladimir Botchkarev and co-workers reveal that the Lim-homeodomain transcription factor Lhx2 differentially regulates these populations during wound healing (p. 4843). They show that, in mice, most of the cells that proliferate in response to skin injury in the HF bulge and secondary hair germ express Lhx2. Wound re-epithelisation is retarded in Lhx2+/– mice compared with wild-type mice, they report, whereas the onset of active hair growth in HFs near to the wound is accelerated. Other experiments indicate that Lhx2 promotes wound re-epithelisation by upregulating Sox9 and Tcf4 expression in the bulge cells while simultaneously inhibiting HF cycling by downregulating Lgr5 expression in the secondary hair germ. Thus, Lhx2 is a key regulator of the differential response of HF stem cells during epidermal regeneration after injury.
Nanog: an ancient reprogrammer
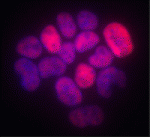
The establishment of pluripotency during mouse embryogenesis and during the reprogramming of somatic cells is dependent on the homeodomain-containing transcription factor Nanog but, puzzlingly, compared with other pluripotency-associated genes, Nanog is poorly conserved among vertebrates. Here (p. 4853), José Silva, Filipe Castro and colleagues investigate whether Nanog orthologues can orchestrate pluripotency in Nanog–/– mouse somatic cells. Surprisingly, the researchers report that mammalian, avian and teleost Nanog orthologues all reprogramme mouse Nanog-/- somatic cells to full pluripotency, despite sharing as little as 13% sequence identity with mouse Nanog. Moreover, they identify two unique residues in the DNA recognition helix of the Nanog homeodomain that are important for reprogramming and show that the Nanog homeodomain is sufficient to enable naive pluripotency in Nanog–/– somatic cells. These functional studies, together with genome analyses, suggest that Nanog is a vertebrate innovation and that its reprogramming capacity resides within a unique DNA-binding domain that probably appeared at least 450 million years ago in a common ancestor of vertebrates.
R-spondin to developmental angiogenesis
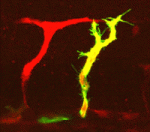
During embryogenesis, two sequential processes form the vasculature: during vasculogenesis, endothelial progenitor cells form the primary vascular bed; subsequently, during angiogenesis, additional vessels sprout and grow from pre-existing vessels. Here, Aniket Gore, Brant Weinstein and co-workers identify a novel signalling pathway that promotes developmental angiogenesis in zebrafish (see p. 4875). Their first clue to this pathway came when they identified a mutation in R-spondin1 (rspo1) during a forward-genetic screen for angiogenesis-deficient zebrafish mutants. Embryos lacking rspo1 or its receptor kremen form primary vessels, they report, but do not undergo angiogenesis. R-spondin is a Wnt signalling regulator and, by functionally manipulating different members of the Wnt pathway, the researchers show that canonical Wnt signalling is required downstream of rspo1 for sprouting angiogenesis. Finally, they show that Vegfc/Vegfr3 signalling mediates the pro-angiogenic effects of Rspo1/Wnt signalling and that all four proteins are expressed by the endothelium during sprouting angiogenesis. Together, these results suggest that Rspo1-Wnt-Vegfc-Vegfr3 signalling is an endothelial-autonomous permissive cue for developmental angiogenesis.
Compartmentalised PKA, cilia and hedgehog signalling
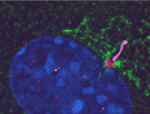
Protein kinase A (PKA), a conserved negative regulator of the hedgehog (Hh) signalling pathway, generates the transcriptional repressor form of Gli3 in the absence of Hh in mice. Now, Kathryn Anderson and colleagues show that the total loss of PKA activity in mouse embryos leads to a completely ventralised neural tube and mid-gestation lethality (see p. 4921), which indicates that the sonic hedgehog (Shh) signalling pathway is maximally activated in all neural progenitors in the absence of PKA. Notably, genetic experiments indicate that the principal function of PKA in the neural plate is to prevent Gli2 activation of Shh targets. Other experiments reveal that Hh pathway activation in PKA mutants depends on cilia, that PKA is localised at the basal body of primary cilia, and that Gli2 levels are increased at the tips of cilia of PKA-null cells. The researchers propose, therefore, that two separate cilia-associated compartments determine the accessibility of Gli proteins to PKA and thus the activity of the Shh pathway in vertebrates.
miR-124 notches up neural development
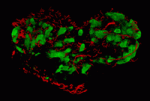
MicroRNAs (miRNAs) play crucial roles in development. miR-124, for example, is abundantly expressed in the mouse brain and is necessary for proper nervous system development, but how it drives neuronal differentiation is unclear. To remedy this lack of understanding, Robert Zeller and colleagues have comprehensively analysed miR-124 expression, function and target genes in the ascidian Ciona intestinalis (see p. 4943). They report that miR-124 interacts with several signalling pathways that are involved in nervous system development. In particular, they show that a feedback interaction between miR-124 and Notch signalling regulates the epidermal-peripheral nervous system (PNS) fate choice in tail midline cells. Thus, Notch signalling silences miR-124 in epidermal midline cells, whereas in PNS midline cells miR-124 silences Notch, Neuralized and the Ciona Hairy/Enhancer-of-Split genes. Moreover, miR-124 also shapes neuronal progenitor fields by downregulating non-neural genes including 50 Brachyury-regulated notochord genes and the muscle specifier Macho-1. Overall, these results indicate that miR-124 plays a multifaceted role in cell lineage specification during nervous system development.
Spotlight on adipogenesis
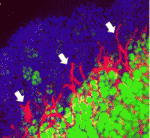
Adipose tissue (a specialised energy storage structure) is the only tissue that can change its mass substantially during adult life. It does this through changes in the size of its constituent cells (adipocytes) and through the de novo generation of cells. Unfortunately, given the obesity epidemic, adipocyte development in vivo is poorly understood but, here, Gou Young Koh and colleagues provide new insights into adipogenesis by analyzing the postnatal development of epididymal adipose tissue (EAT) in mice (p. 5027). They show that EAT is generated from non-adipose tissue during the first 14 postnatal days of development and that this non-adipose tissue is initially composed of multipotent progenitor cells (possibly including adipoblasts) that lack adipogenic differentiation capacity in vitro. By postnatal day 4, however, progenitor cells isolated from EAT can form adipocytes if they are provided with cell-to-matrix and cell-to-cell contacts. Finally, the researchers show that impaired angiogenesis in postnatal mice interferes with adipogenesis. Thus, they conclude, cues from cellular and matrix components, together with appropriate angiogenesis, are required for adipose tissue development.
Plus…
Evolutionary crossroads in developmental biology: amphioxus
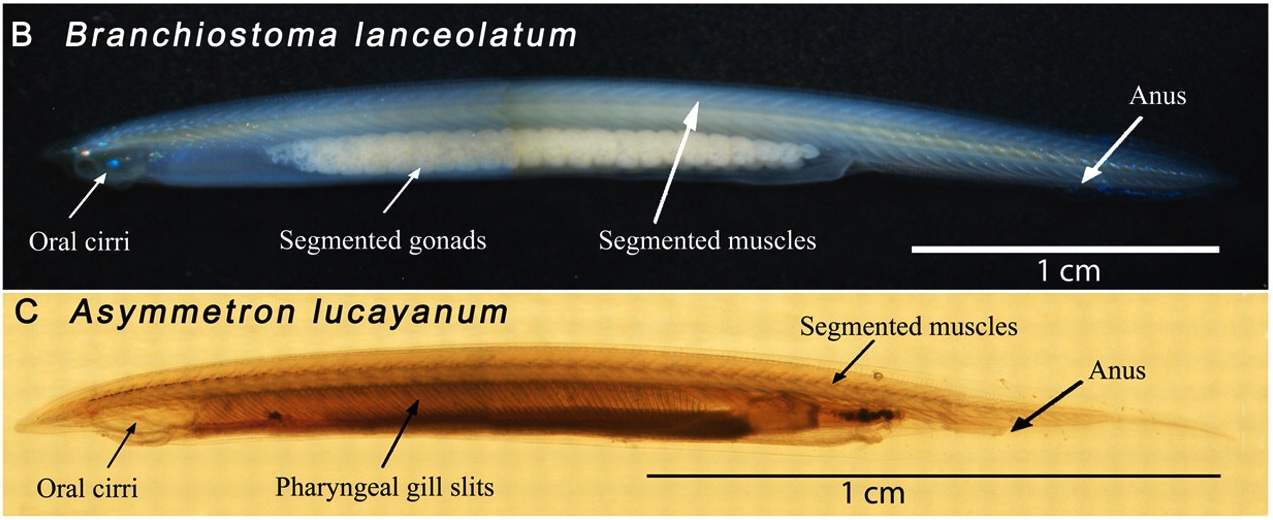
As part of the Evolutionary Crossroads in Developmental Biology series, Bertrand and Escriva introduce amphioxus and discuss how studies of this model have informed us about the evolution of vertebrate traits.
See the Primer article on p. 4819
An interview with Ottoline Leyser

The Sainsbury Laboratory at the University of Cambridge is a new research institute that aims to achieve an integrated understanding of plant development. Its Associate Director is the new plant Editor of Development, Ottoline Leyser, who is also Professor of Plant Development at the University of Cambridge. We recently caught up with Professor Leyser and asked her about the Sainsbury Laboratory and about her own research interests.
See the Spotlight article on p. 4815


 (No Ratings Yet)
(No Ratings Yet)