In Development this week (Vol. 141, Issue 5):
Posted by Seema Grewal, on 18 February 2014
Here are the highlights from the current issue of Development:
Time to update the mammalian CV
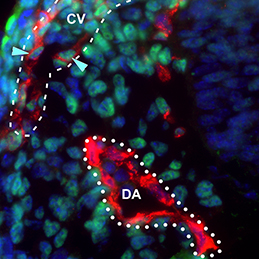 The dorsal aorta (DA) and the cardinal vein (CV) are the first vascular pair to form during development. Although it is generally accepted that the DA derives from angioblasts, the cellular origin of the mammalian CV is currently unknown. Now, on p. 1120, Rong Wang and colleagues reveal that the mammalian CV is formed, at least in part, from endothelial cells that originate from within the DA. Using markers specific to either aortic or venous-fated endothelial cells, the authors show that the DA contains a mixed population of endothelial cells with either venous or arterial identity, but that the number of venous-fated endothelial cells decreases over time. The authors use time-lapse microscopy to visualise the migration of endothelial cells away from the DA and to the CV, and further show that this process requires ephrin B2/EphB4 signalling. The authors propose a mechanism whereby ephrin B2/EphB4-mediated cell repulsion drives the segregation of venous-fated endothelial cells away from the DA, facilitating their movement to the CV.
The dorsal aorta (DA) and the cardinal vein (CV) are the first vascular pair to form during development. Although it is generally accepted that the DA derives from angioblasts, the cellular origin of the mammalian CV is currently unknown. Now, on p. 1120, Rong Wang and colleagues reveal that the mammalian CV is formed, at least in part, from endothelial cells that originate from within the DA. Using markers specific to either aortic or venous-fated endothelial cells, the authors show that the DA contains a mixed population of endothelial cells with either venous or arterial identity, but that the number of venous-fated endothelial cells decreases over time. The authors use time-lapse microscopy to visualise the migration of endothelial cells away from the DA and to the CV, and further show that this process requires ephrin B2/EphB4 signalling. The authors propose a mechanism whereby ephrin B2/EphB4-mediated cell repulsion drives the segregation of venous-fated endothelial cells away from the DA, facilitating their movement to the CV.
Mammary stratification caught on camera
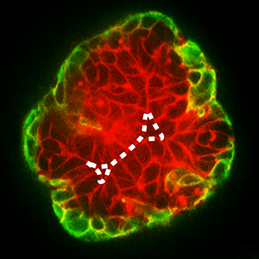 During development, select epithelial cells must undergo asymmetric division in order to generate a stratified epithelium. Previous studies have shown that basally positioned epithelial stem cells orchestrate this process, but the mammary epithelium has two distinct cell layers inside the basement membrane, so it remains unclear which cell type is responsible for stratification. Using elegant time-lapse imaging of three-dimensional mouse mammary epithelial cultures, Andrew Ewald and colleagues now reveal (p. 1085) that it is the apical luminal epithelial cells that divide vertically to generate the stratified mammary epithelium. The authors show that this is accompanied by the loss of tight junctions, as marked by ZO-1, as well as loss of apicobasal polarity in the new daughter cells. Importantly, the authors also demonstrate that this mechanism of stratification and loss of polarity operates during early oncogenesis in the mouse mammary epithelium. These data uncover a common cellular mechanism that underpins the developmental and oncogenic stratification of mammary tissue.
During development, select epithelial cells must undergo asymmetric division in order to generate a stratified epithelium. Previous studies have shown that basally positioned epithelial stem cells orchestrate this process, but the mammary epithelium has two distinct cell layers inside the basement membrane, so it remains unclear which cell type is responsible for stratification. Using elegant time-lapse imaging of three-dimensional mouse mammary epithelial cultures, Andrew Ewald and colleagues now reveal (p. 1085) that it is the apical luminal epithelial cells that divide vertically to generate the stratified mammary epithelium. The authors show that this is accompanied by the loss of tight junctions, as marked by ZO-1, as well as loss of apicobasal polarity in the new daughter cells. Importantly, the authors also demonstrate that this mechanism of stratification and loss of polarity operates during early oncogenesis in the mouse mammary epithelium. These data uncover a common cellular mechanism that underpins the developmental and oncogenic stratification of mammary tissue.
An epigenetic bag of TRX
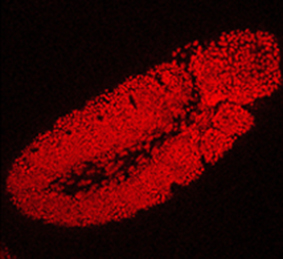 The Drosophila Trithorax (TRX) protein plays a key role in maintaining active transcription of many master cell fate regulatory genes. Previously, TRX was thought to function mainly at the promoter by depositing the histone H3K4 trimethylation mark (H3K4me3) via its catalytic SET domain. However, recent findings have challenged this view, prompting new investigations into the function of TRX. Now, on p. 1129, Peter Harte, Feng Tie and colleagues reveal that TRX, along with TRX-related (TRR), is responsible for histone H3K4 monomethylation (H3K4me1), and not trimethylation, suggesting a role for TRX in stimulating enhancer-dependent transcription. In vivo studies support these findings, as a catalytically inactive form of TRX results in reduced H3K4me1, but no change in H3K4me3, in Drosophila embryos. The authors also show that TRX collaborates directly with CREB-binding protein (CBP) to promote robust H3K27 acetylation, which antagonises Polycomb silencing and may also stimulate enhancer-dependent transcription. These data provide exciting new insights into the mechanisms of epigenetic regulation in developing organisms.
The Drosophila Trithorax (TRX) protein plays a key role in maintaining active transcription of many master cell fate regulatory genes. Previously, TRX was thought to function mainly at the promoter by depositing the histone H3K4 trimethylation mark (H3K4me3) via its catalytic SET domain. However, recent findings have challenged this view, prompting new investigations into the function of TRX. Now, on p. 1129, Peter Harte, Feng Tie and colleagues reveal that TRX, along with TRX-related (TRR), is responsible for histone H3K4 monomethylation (H3K4me1), and not trimethylation, suggesting a role for TRX in stimulating enhancer-dependent transcription. In vivo studies support these findings, as a catalytically inactive form of TRX results in reduced H3K4me1, but no change in H3K4me3, in Drosophila embryos. The authors also show that TRX collaborates directly with CREB-binding protein (CBP) to promote robust H3K27 acetylation, which antagonises Polycomb silencing and may also stimulate enhancer-dependent transcription. These data provide exciting new insights into the mechanisms of epigenetic regulation in developing organisms.
Oct4: the plot thickens
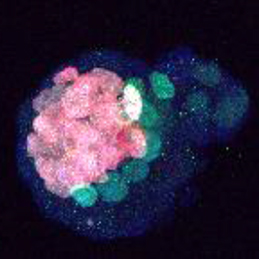 The transcription factor Oct4 is well known for its role in maintaining pluripotency in vitro and for preventing ectopic differentiation of early embryos in vivo. Recent evidence also suggests a role for Oct4 in lineage specification; however, little is known about how this occurs and the manner in which Oct4 is required. Now, on p. 1001, Jennifer Nichols and colleagues report a non-cell-autonomous requirement for sustained Oct4 expression during primitive endoderm (PrE) specification. The authors confirm that maternal and zygotic Oct4 are not required for development to the blastocyst stage, but that conditional inactivation of Oct4 in the early blastocyst results in reduced expression of PrE markers Sox17 and Gata4, and a failure to generate PrE-derived tissue in chimeric assays. Surprisingly, the formation of PrE can be rescued if the conditionally inactivated Oct4 mutants are injected at the pre-blastocyst stage with wild-type embryonic stem cells, suggesting that Oct4 is not required to operate cell-autonomously in order to specify the PrE.
The transcription factor Oct4 is well known for its role in maintaining pluripotency in vitro and for preventing ectopic differentiation of early embryos in vivo. Recent evidence also suggests a role for Oct4 in lineage specification; however, little is known about how this occurs and the manner in which Oct4 is required. Now, on p. 1001, Jennifer Nichols and colleagues report a non-cell-autonomous requirement for sustained Oct4 expression during primitive endoderm (PrE) specification. The authors confirm that maternal and zygotic Oct4 are not required for development to the blastocyst stage, but that conditional inactivation of Oct4 in the early blastocyst results in reduced expression of PrE markers Sox17 and Gata4, and a failure to generate PrE-derived tissue in chimeric assays. Surprisingly, the formation of PrE can be rescued if the conditionally inactivated Oct4 mutants are injected at the pre-blastocyst stage with wild-type embryonic stem cells, suggesting that Oct4 is not required to operate cell-autonomously in order to specify the PrE.
RAd migration in the cortex
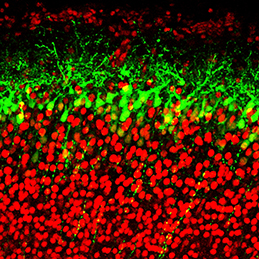 The generation of layer-specific cortical neurons is fundamental to the circuitry of the developing and adult brain. Although the intrinsic drivers of neuronal specification are becoming increasingly understood, the extrinsic signals that guide migration and consolidate post-mitotic neuronal identity are less clear. In this issue (p. 1151), Shanthini Sockanathan and colleagues investigate the role of endogenous retinoic acid (RA) signalling in regulating the radial migration and laminar fate of post-mitotic cortical neurons. Using a dominant-negative RA receptor construct, the authors show that ablation of RA signalling in mouse embryos in utero not only delays migration of subsets of cortical neurons, but also results in a failure to maintain their correct regional identity following migration. This phenotype can be partially rescued by stabilised β-catenin, which the authors show is normally maintained by RA signalling. This work sheds light on the extrinsic mechanisms that control cortical neuronal development and has important implications for disorders in which cortical neuronal circuitry is de-regulated.
The generation of layer-specific cortical neurons is fundamental to the circuitry of the developing and adult brain. Although the intrinsic drivers of neuronal specification are becoming increasingly understood, the extrinsic signals that guide migration and consolidate post-mitotic neuronal identity are less clear. In this issue (p. 1151), Shanthini Sockanathan and colleagues investigate the role of endogenous retinoic acid (RA) signalling in regulating the radial migration and laminar fate of post-mitotic cortical neurons. Using a dominant-negative RA receptor construct, the authors show that ablation of RA signalling in mouse embryos in utero not only delays migration of subsets of cortical neurons, but also results in a failure to maintain their correct regional identity following migration. This phenotype can be partially rescued by stabilised β-catenin, which the authors show is normally maintained by RA signalling. This work sheds light on the extrinsic mechanisms that control cortical neuronal development and has important implications for disorders in which cortical neuronal circuitry is de-regulated.
Maternal undernutrition stimulates embryo endocytosis
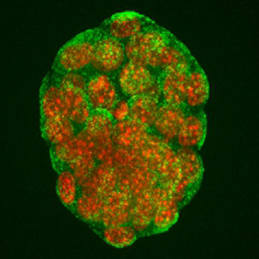 The production of healthy offspring depends on many factors spanning from intrinsic genetic elements to variations in the in uteroenvironment. Poor maternal diet is associated with increased risk of cardiovascular, metabolic and behavioural disorders during later life of the offspring, but how the developing embryo copes with maternal dietary stress has not been well characterised. Now, on p. 1140, Tom Fleming and colleagues investigate the compensatory mechanisms that are activated when the early embryo is challenged by poor maternal nutrition. Using quantitative imaging techniques and extensive marker analyses, the authors show that a restricted-protein maternal diet results in stimulated endocytosis within both the trophectoderm and the primitive endoderm of the early mouse embryo to overcome the shortfall in nutrient supply. The authors show that enhanced trophectoderm endocytosis occurs in response to reduced branched-chain amino acids and is mediated via RhoA GTPase signalling. This exciting finding is an important step in uncovering the cellular mechanisms that underpin disorders caused by poor maternal nutrition.
The production of healthy offspring depends on many factors spanning from intrinsic genetic elements to variations in the in uteroenvironment. Poor maternal diet is associated with increased risk of cardiovascular, metabolic and behavioural disorders during later life of the offspring, but how the developing embryo copes with maternal dietary stress has not been well characterised. Now, on p. 1140, Tom Fleming and colleagues investigate the compensatory mechanisms that are activated when the early embryo is challenged by poor maternal nutrition. Using quantitative imaging techniques and extensive marker analyses, the authors show that a restricted-protein maternal diet results in stimulated endocytosis within both the trophectoderm and the primitive endoderm of the early mouse embryo to overcome the shortfall in nutrient supply. The authors show that enhanced trophectoderm endocytosis occurs in response to reduced branched-chain amino acids and is mediated via RhoA GTPase signalling. This exciting finding is an important step in uncovering the cellular mechanisms that underpin disorders caused by poor maternal nutrition.
PLUS…
Cell competition: how to eliminate your neighbours
 It has been known for years, based on studies of Drosophila, that viable cells can be eliminated by their neighbours through a process termed cell competition. New studies in mammals have revealed that this process is universal and that many factors and mechanisms are conserved. Here, Amoyel and Bach provide an overview of the mechanistic steps involved in cell competition and discuss recent advances in the field, which have shed light on how and why cell competition exists in developing and adult organisms. See the Review on p. 988
It has been known for years, based on studies of Drosophila, that viable cells can be eliminated by their neighbours through a process termed cell competition. New studies in mammals have revealed that this process is universal and that many factors and mechanisms are conserved. Here, Amoyel and Bach provide an overview of the mechanistic steps involved in cell competition and discuss recent advances in the field, which have shed light on how and why cell competition exists in developing and adult organisms. See the Review on p. 988
The Mediator complex: a master coordinator of transcription and cell lineage development
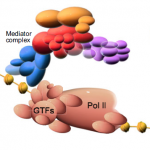 Mediator is a multiprotein complex that is required for gene transcription by RNA polymerase II. Here, Yin and Wang describe the most recent advances in understanding the mechanisms of Mediator function, with an emphasis on its role during development and disease. See the Primer on p. 977
Mediator is a multiprotein complex that is required for gene transcription by RNA polymerase II. Here, Yin and Wang describe the most recent advances in understanding the mechanisms of Mediator function, with an emphasis on its role during development and disease. See the Primer on p. 977


 (No Ratings Yet)
(No Ratings Yet)