In Development this week (Vol. 142, Issue 15)
Posted by Seema Grewal, on 4 August 2015
Here are the highlights from the current issue of Development:
CHD4 restrains gene expression during lineage specification
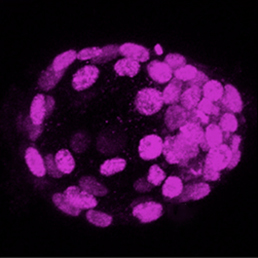
A neuronal ballet mediates the lamination of the retina
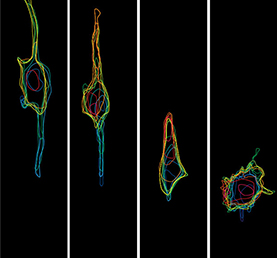
In the mature retina, retinal inhibitory neurons (RINs) are arranged in three distinct layers composed of horizontal cells (HCs), inner nuclear layer amacrine cells (iACs) and displaced amacrine cells (dACs), respectively. How do such interneurons reach their specific laminar positions during development? To explore this question, William Harris and co-workers (p.2665) quantify cell behaviour in the developing retina of several transgenic zebrafish lines over long periods of time. They first show that all RIN types show a bipolar morphology and migrate to the centre of the retina, near the region where the inner plexiform layer (IPL) later forms. RINs then adopt a multipolar morphology and can migrate tangentially, frequently changing direction. Interestingly, multipolar RINs are highly dynamic and do not just pile up in the centre of the retina according to their time of arrival, as previously thought. Moreover, RINs undergo cell type-specific behaviours that fine-tune their position. Contrary to previous belief, dACs actively migrate to their respective layer through the proto-IPL rather than being trapped in their layer by the future IPL. This study offers a valuable framework for further dissecting the molecular mechanisms of retina lamination.
Shaping long-term hematopoietic stem cells
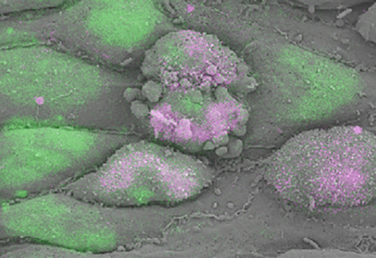
During embryogenesis, a subset of endothelial cells, the haemogenic endothelium, undergoes an endothelial-to-hematopoietic transition (EHT) to generate the first long-term haematopoietic stem cells, which bud off the aorta – a known haemogenic site – as intra-aortic clusters. On p.2719, Frank Bos et al. use correlative scanning electron microscopy to associate the morphological changes seen in murine and human haemogenic endothelium during EHT with the expression of RUNX1 and SOX17, two transcription factors required for EHT. During this process, the authors observe that in a subset of initially flat, smooth and oblong endothelial cells with low RUNX1/SOX17 ratio, the acquisition of a haematopoietic fate, identified by the expression of CD41 and c-kit, is associated with an increase in RUNX1/SOX17 ratio, the acquisition of a rounded morphology and the appearance of previously undescribed filopodia-like structures at the cell membrane. This innovative method allows the analysis of EHT a single-cell level and sheds light on the morphological changes associated with the acquisition of haematopoietic fate.
Building a kidney through Fat/Dchs-mediated cell-communication
Formation of the adult kidney requires interactions between three adjacent cell populations of the embryonic kidney: the epithelial ureteric bud (UB), the cap mesenchyme (CM), which gives rise to nephron progenitors, and the stromal mesenchyme (stroma). How these different cell types communicate to achieve successful kidney morphogenesis is not yet fully understood. In this issue, two studies assess the contribution of Fat4 and Dchs1/2, members of the cadherin family that regulate both Hippo and planar cell polarity signalling, to the cell-cell communication required for kidney development.
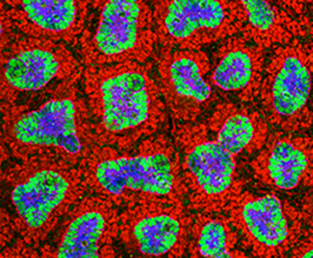 On p.2574, Kenneth Irvine and colleagues found that, as previously described for Fat4 mutant mice, the ablation of Dchs1 leads to an expansion of the nephron progenitor pool, indicating that Fat4 and Dchs1 function as signalling partners. Interestingly, and contrary to previous studies, the authors did not observe activation of Yap in association with the CM expansion caused by Fat4 or Dchs1 depletion. Using conditional deletion of Dchs1 in different kidney populations, the authors show that Dchs1 is specifically required in CM cells, where it localizes to the cell surface that contact the stroma, to regulate their differentiation, the development of the stroma and, indirectly, that of the UB. This study sheds light on the cellular and molecular mechanisms of the cell-cell communication that orchestrates kidney morphogenesis.
On p.2574, Kenneth Irvine and colleagues found that, as previously described for Fat4 mutant mice, the ablation of Dchs1 leads to an expansion of the nephron progenitor pool, indicating that Fat4 and Dchs1 function as signalling partners. Interestingly, and contrary to previous studies, the authors did not observe activation of Yap in association with the CM expansion caused by Fat4 or Dchs1 depletion. Using conditional deletion of Dchs1 in different kidney populations, the authors show that Dchs1 is specifically required in CM cells, where it localizes to the cell surface that contact the stroma, to regulate their differentiation, the development of the stroma and, indirectly, that of the UB. This study sheds light on the cellular and molecular mechanisms of the cell-cell communication that orchestrates kidney morphogenesis.
In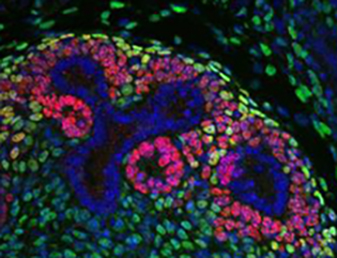 the second study (p.2564), Helen McNeill and co-workers show that in the stroma, Fat4 (but not Fat1 or Fat3) interacts with Dchs1 and 2 (which act partially redundantly) to regulate CM differentiation. Interestingly, they further show that Fat4 regulates the nephron progenitor pool size independently of YAP and the core PCP Vangl2, but through Six2, a known regulator of CM size. Additionally, the authors find that Fat4 loss leads to defective cellular organization of the CM and UB-derived tubules and to the alteration of Notch and FGF pathways, providing further insight into the molecular mechanisms governing the intercellular communications that mediate kidney morphogenesis.
the second study (p.2564), Helen McNeill and co-workers show that in the stroma, Fat4 (but not Fat1 or Fat3) interacts with Dchs1 and 2 (which act partially redundantly) to regulate CM differentiation. Interestingly, they further show that Fat4 regulates the nephron progenitor pool size independently of YAP and the core PCP Vangl2, but through Six2, a known regulator of CM size. Additionally, the authors find that Fat4 loss leads to defective cellular organization of the CM and UB-derived tubules and to the alteration of Notch and FGF pathways, providing further insight into the molecular mechanisms governing the intercellular communications that mediate kidney morphogenesis.
PLUS:
An interview with Lewis Wolpert
 We recently met with Lewis Wolpert at the Spring Meeting of the British Society for Developmental Biology, where he was awarded the Waddington Medal, and asked him about his life and his career. See the Spotlight article on p. 2547
We recently met with Lewis Wolpert at the Spring Meeting of the British Society for Developmental Biology, where he was awarded the Waddington Medal, and asked him about his life and his career. See the Spotlight article on p. 2547
Transcriptional and epigenetic insights from stem cells and developing tissues
In this meeting review, Daniel Lim discusses major advances in our understanding of the epigenetic and transcriptional regulation of stem cell states, presented at a recent Keystone Symposium. See the Meeting Review on p. 2549
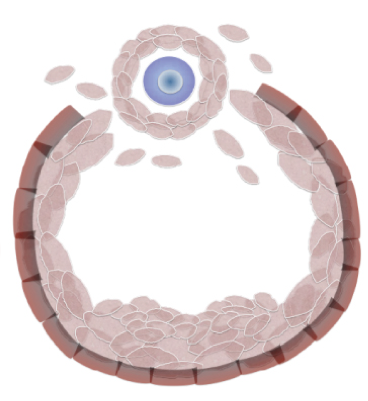 The developmental origins of the mammalian ovarian reserve
The developmental origins of the mammalian ovarian reserve
Grive and Freiman discuss the importance of the formation and survival of the primordial follicle pool during fetal and neonatal periods for the long-term reproductive capacity of female mammals. See the Review on p. 2554
Featured movie
Our latest featured movie shows the movements of the cytoplasm within a normal zebrafish embryo. In their latest paper, Solnica-Krezel and colleagues showed that these movements are impaired in dchs1b mutants, suggesting defects in the actin cytoskeleton. See the paper on p.2704


 (No Ratings Yet)
(No Ratings Yet)