Making time matter: how hormone pulses direct chromatin accessibility during development
Posted by Matt Niederhuber, on 8 August 2017
Each of our cells has the same genetic information and thus the same potential to become a part of a heart, brain, or a finger. Somehow though, during development our cells manage to figure out exactly which type of cell they should be and which body parts they should help compose. The key to making this work is precise control over gene expression, such that as a single cell divides to make trillions, the correct genes are turned on and off at precisely the right times and places. When gene regulation fails, developmental disorders and diseases like cancer can occur.
Today, transcriptional regulation in animals is understood to involve a complex integration of cis-regulatory elements (enhancers), cell signaling pathways, nucleosome occupancy, and higher order chromatin architecture that all work in concert to direct gene expression. In the McKay Lab we are interested in how spatial and temporal regulatory information is integrated during development, and how that integration produces the distinct cell types and body parts of animals.
Central to the process of differential gene regulation are relatively small genomic regions called enhancers. Enhancers, as their name implies, have long been known to increase gene expression, often over long distances. In extreme cases an enhancer can act on a gene that is over 1 million bases away, such as in the case of the enhancer that regulates the Sonic hedgehog gene1.
Enhancers mediate gene activation by serving as landing pads for proteins (transcription factors) that recruit the machinery required for gene transcription. Enhancers are remarkable not only for their ability to regulate gene expression across long genomic distances, but also because they exhibit highly flexible sequence characteristics, they work independently of their orientation, and they can be transplanted to different parts of the genome and still retain their ability to activate nearby genes2. The role and importance of enhancers has become increasingly apparent, especially as the links between human disease and mutations within enhancers have become much clearer3-5.
One of the major current question in the field is how networks of enhancers direct the differential gene expression programs during development.
Inside eukaryotic cells, DNA is packaged into chromatin, the basic unit of which is the nucleosome. Wrapping DNA around nucleosomes succeeds in packaging two meters of DNA into a ten-micron nucleus. It also serves as a potential means of controlling access to the DNA sequence because nucleosomes typically block transcription factors from binding their target enhancers. For a transcription factor to bind DNA, its target site must be sufficiently depleted of nucleosomes or “opened.” Consequently, one hypothesis for how transcription factor binding is controlled (and hence for determining which enhancers are active) is through regulation of chromatin accessibility. According to this model, an enhancer with cell-type specific activity would be open in cells in which it is active, but closed in cells in which it is inactive (Figure 1).
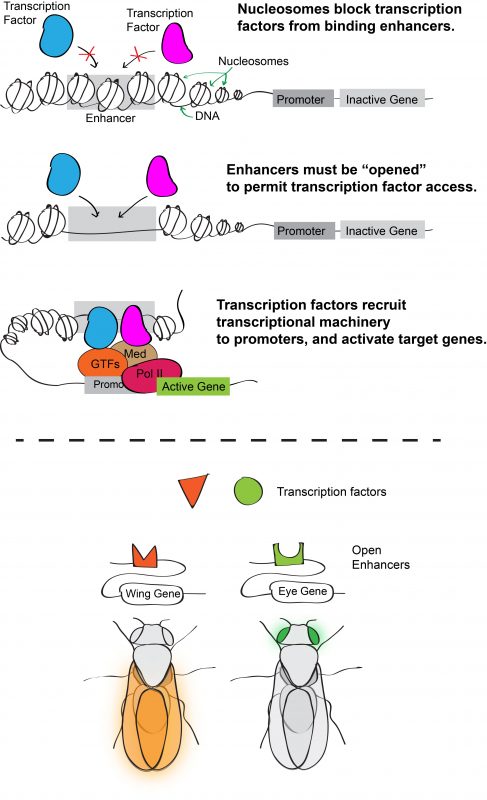
During his postdoctoral work, our PI Dan McKay tested this idea by profiling the open chromatin regions during appendage development in the fruit fly Drosophila melanogaster6. Using a high-throughput sequencing method that identifies genomic regions of low nucleosome occupancy, called FAIRE-Seq, Dan found that, despite having different morphologies, transcriptional programs, and transcription factors that specify the distinct identity of each appendage (so called master transcription factors), the patterns of open chromatin were surprisingly similar between the cells of wings, legs, and halteres. Even more surprising was the finding that although these patterns were dynamic over time, they remained highly similar between the appendages. In other words, the same regions of the genome appeared to be opening and closing at similar times, even though the cells were in completely different parts of the animal. This indicated that the temporal control of chromatin accessibility might be a more significant driver of differential gene expression during appendage development than spatial control (Figure 2).
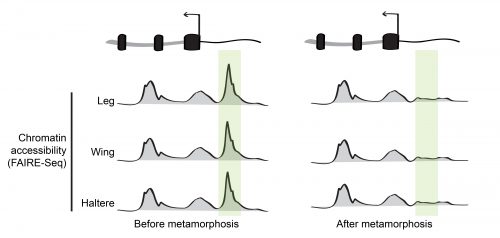
Dan’s 2013 paper raised a big question: If the transcription factors that specify the distinct identity of each appendage weren’t primarily responsible for directing chromatin accessibility, what exactly was? And on top of that, how were these changes in open chromatin being coordinated across spatially-separated tissues?
One of the phenomenal things about insects is that the timing of their development is precisely controlled by a steroid hormone called ecdysone. In Drosophila, levels of ecdysone increase at stereotypical developmental timepoints, particularly during major transitions like molting7. The work of Michael Ashburner in the 1970s revealed that these ecdysone pulses were responsible for activating a large set of genes, many of which were eventually found to be DNA-binding transcription factors8. Because ecdysone acts systemically, and it has a known role in controlling gene expression, we wondered if ecdysone, and in turn ecdysone-induced proteins, contributed to the coordinated changes in chromatin accessibility that Dan had observed.
Focusing our attention on wing development, our first step was to get a better sense of how chromatin accessibility was changing in the wing9. Our original FAIRE data had only looked at chromatin accessibility at two time points: a stage right before metamorphosis and a much later stage at the end of metamorphosis. Consequently, it was essential to get FAIRE-Seq data in the pupal wing at finer timepoints. With collaboration from the Buttitta Lab at the University of Michigan, we obtained FAIRE-Seq data that confirmed Dan’s observations; chromatin accessibility was changing significantly, with thousands of regions opening and closing over a relatively brief two-day period. Based on these results we knew that the pupal wing, which undergoes striking morphological changes as it develops, was going to be an excellent model system for us to examine how chromatin access is regulated.
Since ecdysone is a steroid hormone and is essential for even the earliest stages of fly development, we couldn’t remove it completely. Instead, we focused our attention on one of the transcription factors directly induced by ecdysone, Eip93F (E93). To ask whether E93 was involved in changing chromatin accessibility, we repeated our FAIRE-seq experiments in an E93 mutant fly. We found that many regions (~50%) that originally showed dynamic changes in accessibility, failed to change their state in the mutant. So, it appeared that we were on the right track and that E93, and by proxy ecdysone signaling, was required for directing many genome-wide changes in chromatin accessibility. Importantly, our data showed that E93 was required for both opening and closing of chromatin.
Although these data showed us that E93 was required for changes in chromatin accessibility, it didn’t tell us whether it was directly causing these changes. To help answer this question, we wanted to determine where E93 was physically bound in the genome. These days assaying protein-DNA binding by ChIP-Seq is a standard practice, but the method requires a significant amount of input to get good results. When working with cell culture this isn’t much of a problem, but it presents a serious challenge when working with tissues in tiny animals like Drosophila, because we needed over a thousand wings to have sufficient input. Over the course of a week, the lab worked together to dissect all the wings necessary for the experiment, and in the end the effort paid off. We found that not only was E93 required for many of the changes in chromatin accessibility, it actually bound to about half of these dynamic regions, arguing that in many cases E93 had a direct role in coordinating both opening and closing chromatin.
Next, we had to tackle whether the temporally dynamic chromatin regions we observed in our FAIRE data actually corresponded to functional enhancers. The best method for testing enhancer functionality is still to clone them upstream of a fluorescent reporter gene. This can work great, but makes it much harder to test many candidates with a high degree of throughput, as it can take months to establish and test the transgenic flies. This was right around the time I joined the lab as a first-year graduate student, and I got the chance to work on characterizing the function of several of the putative enhancers we had cloned. We were thrilled to find that the E93 dependent dynamic chromatin regions we had cloned successfully drove GFP expression, and that the timing of their activity correlated with the timing of the accessibility of the native enhancer.
The crux of this project was to finally test if the failures that we saw in enhancers opening or closing in E93 mutants correlated with a functional defect in our reporters. We found that in the absence of E93 there were dramatic changes in enhancer activity, and that these changes followed what we would expect based on the changes we saw in chromatin accessibility. For example, the nubvein enhancer, which normally opens during pupal wing development, produces striking patterns of fluorescence along the wing veins when it’s cloned upstream of a GFP gene. However, in an E93 mutant fly, this region fails to open to the same degree. When we imaged the nubvein enhancer reporter in the presence of E93 RNAi we saw complete loss of the normal vein pattern of expression (Figure 3).
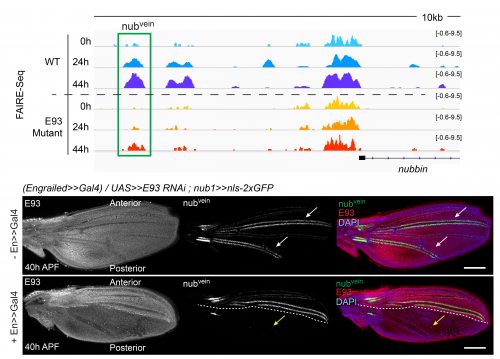
What we found most exciting about this project is that it demonstrated that an extrinsic signal, ecdysone, which pulses at specific times in development, alters the accessibility of enhancers to binding by transcription factors. In other words, for a genome that contains far greater regulatory capacity than is used at a given point in time, hormone signaling can help to determine which subsets of the genome are accessible for use at given time in development. Thus, ecdysone-regulated chromatin accessibility provides a temporal-specific input, which is combined with spatial-specific input in the form of tissue-specific transcription factors. Amazingly, both forms of input are integrated by enhancers.
This has left us with a whole new set of questions to go after. What are the mechanisms that drive ecdysone-dependent changes in chromatin accessibility? Does E93 act alone? How is it that E93 is required for both opening and closing chromatin? How does chromatin accessibility fit into other aspects of gene regulation such as long-distance enhancer-promoter interactions? There’s a lot more to figure out, and these questions are actively pushing us to expand our research focus. Fortunately for me, that means there’s a lot of room to explore and opportunity to make some meaningful contributions to the field.
References
1. Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. (2003). A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 12(14):1725-35.
2. Shlyueva D, Stampfel G, Stark A. (2014). Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev. 15:272-286.
3. Maurano M, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. (2012). Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Sci. 337(6099):1190-1195.
4. Visel A, Rubin EM, Pennacchio LA. (2009). Genomic views of distant-acting enhancers. Nat. 461:199-205.
5. Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. (2005). Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Dev. 132:797-803.
6. McKay DJ, Lieb JD. (2013). A common set of DNA regulatory elements shapes Drosophila appendages. Dev Cell. 27:306-318.
7. Richards G. (1981). The radioimmune assay of ecdysteroid titres in Drosophila melanogaster. Mol. and Cell. Endo. 21:181-197.
8. Ashburner M. (1974). Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. Dev. Bio. 39:141-157.
9. Christopher M. Uyehara, Spencer Nystrom, Matthew J. Niederhuber, Mary Leatham-Jensen, Yiqin Ma, Laura A. Buttitta & Daniel J. McKay (2017). Hormone-dependent control of developmental timing through regulation of chromatin accessibility. Genes & Development. 31:862-875.


 (2 votes)
(2 votes)