Molecular study of changing expression of photoreceptor genes throughout development of Anopheles gambiae
Posted by hannah cowling, on 18 December 2022
Hannah Cowling, MBiol at Durham University
Introduction
Over summer 2022, I had the opportunity to work with Dr Olena Riabinina in Insect Neuro Lab at Durham University. Her team specialises in neurobiology and neuroecology of insects with established work in mosquito olfaction and bumblebee olfactory neurobiology and ecology. My project was working with PhD student Matthew Quinn, assisting with a chapter of his PhD research project to molecularly characterise the development of the larval visual systems in the Anopheles gambiae mosquito.
Anopheles gambiae are a group of species which include the most significant vectors of deadly disease malaria in sub-Saharan Africa. As malaria poses one of the most significant public health threats worldwide, research surrounding mosquito sensory systems could prove vital in informing vector management techniques. One avenue of Insect Neuro Lab’s previous research has focussed on mosquito olfaction. Significant because research indicates sense of smell is crucial in host seeking behaviour (Wheelwright et al., 2021)(Riabinina et al., 2016). However, as mosquito larvae and pupae are also responsive to visual stimuli, a deeper understanding of their visual development has potential to provide valuable methods of mosquito-driven disease prevention. Visual perception of environmental cues is crucial for insects to, in combination with other senses, avoid predators, source food, mates and ovipositioning.
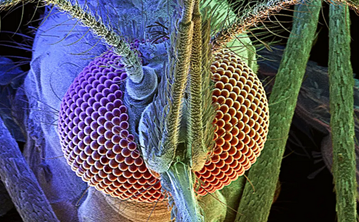
Compared to the mammalian ‘single-aperture’ eye, mosquitos have poor image resolution, however, can detect comparatively fast movement in a large viewing angle. Each unit is composed of a cornea, lens, and photoreceptor cells which sense light wavelength (colour) and intensity. Opsins are a highly conserved photoreceptor molecules observed in mosquitos and across the animal kingdom. They are membrane-bound proteins which absorb photons and change their conformation, initiating a signalling cascade known as phototransduction (Shichida & Matsuyama, 2009). Previous studies have shown that An. gambiae have 11 opsin genes, 6 of which detect long wave light. This is more than typical for insects which usually have 4. There has been a suggested association of increased long wave opsins being found to accommodate more complex light conditions within aquatic environments (Giraldo-Calderón et al., 2017).
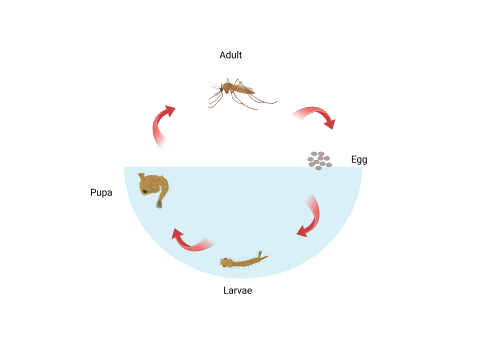
Shown in figure 2, mosquitos have four different stages in their life cycle: Egg, larvae, pupa, and adult. For the larval and pupal stages of development, mosquitos live in an aquatic environment. In contrast, adults are flying insects. It follows that their visual systems will be differently adapted to better assist survival in the contrasting environments. Previous studies involving the larvae of dengue and yellow fever transmitting mosquito Aedes aegypti show that mosquitos develop adult eye cells in late larval and pupal stages. It is predicted that both sets of eyes contribute to visual capabilities but have different photoreceptor cell types (Mysore et al., 2014). This difference in gene expression over time enables genetic targeting and quantification of components of the two visual systems. The aim of this project was to use molecular techniques to characterise expression of opsin genes in larval and pupal An. gambiae and to test the hypothesis that opsin expression profile changes throughout development.
Methods
The project involved synthesizing cDNA from RNA extracted from An. Gambiae at different time stages ranging from egg to adult. CDNA synthesis was necessary because amplification of RNA requires conversion into double stranded form. This was done using a kit containing Moloney murine leukaemia virus reverse transcriptase. The resulting cDNA was used to perform qPCR using ten different opsin and two housekeeping genes as templates. The method utilised real-time fluorescence of a double stranded DNA binding dye to detect amplification at each cycle of PCR. When fluorescent signal is detected above a decided threshold of background fluorescence, a quantification value/ Cq value is determined which calculates relative abundance between samples.
Results
We found that, consistent with previous findings (Jenkins & Muskavitch, 2015), expression of long wavelength-sensing opsin genes was distinct between larval and adult stages. With notably high expression of Opsin 6 in larval stages and high expression of opsins 1,3 and 4 in adult An. gambiae. Opsin 8 which encodes an ultraviolet sensing photoreceptor showed expression in both larval and adult forms which slightly increased in the latter. Lastly, opsin 9 which detects short-wavelength light had very low expression in the early larval stages increasing steadily with time into the adult. This research will be followed up with behavioural assays using knockout mutants to ascertain the roles of the different opsin genes in larvae survival behaviour.
Conclusions and Looking Forward
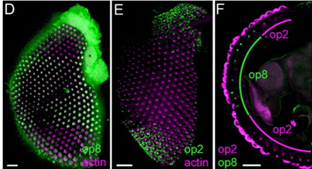
If I were to have the opportunity to pursue this research further, I would be interested to conduct immunolocalization imaging on larvae and pupae retinas comparative to research seen in figure 3 on adult An. gambiae. To map the different opsin gene expression of the larval and pupal eye structures would shed insight into how the expression levels observed translates structurally.
The project taught me much about experimental design at a PhD level. As I am considering a career in research, it was incredibly useful for me to observe this in action. I understand better how to seek and try to fill a gap in the literature and how to use a range of molecular techniques to test my hypothesis. Furthermore, the project gave me a lot of confidence in the lab and in my own abilities. After studying for the first two years of my bachelors in the climate of covid, our laboratory time had been limited and we spent much of our studies at home watching our lectures on our computers. Hence, experiencing two months of being a part of a friendly and welcoming lab community was so good for me. Under Matthew’s patient tutelage I added some fundamental molecular skills to my repertoire and deepened my understanding of an area of interest. This knowledge will undoubtably be useful for my degree modules this year and certainly for my level 4 lab-based research year. Additionally, because of being allowed to do this project I have chosen to do my level 3 literature review module within the subject areas of insect neurology and development. The summer has sparked an interest in new areas for me and shown me the benefits of insects as model organisms. Many thanks to everyone at Insect Neuro Lab and the BSDB for facilitating this experience.
Giraldo-Calderón, G. I., Zanis, M. J., & Hill, C. A. (2017). Retention of duplicated long-wavelength opsins in mosquito lineages by positive selection and differential expression. BMC Evolutionary Biology, 17(1). https://doi.org/10.1186/s12862-017-0910-6
Hu, X., England, J. H., Lani, A. C., Tung, J. J., Ward, N. J., Adams, S. M., Barber, K. A., Whaley, M. A., & O’Tousa, J. E. (2009). Patterned rhodopsin expression in R7 photoreceptors of mosquito retina: Implications for species-specific behavior. The Journal of Comparative Neurology, 516(4), 334–342. https://doi.org/10.1002/cne.22114
Jenkins, A. M., & Muskavitch, M. A. T. (2015). Crepuscular behavioral variation and profiling of opsin genes in Anopheles gambiae and Anopheles stephensi (diptera: Culicidae). Journal of Medical Entomology, 52(3), 296–307. https://doi.org/10.1093/jme/tjv024
Mysore, K., Flannery, E., Leming, M. T., Tomchaney, M., Shi, L., Sun, L., O’Tousa, J. E., Severson, D. W., & Duman-Scheel, M. (2014). Role of semaphorin-1a in the developing visual system of the disease vector mosquito Aedes aegypti. Developmental Dynamics, 243(11), 1457–1469. https://doi.org/10.1002/dvdy.24168
Riabinina, O., Task, D., Marr, E., Lin, C. C., Alford, R., O’Brochta, D. A., & Potter, C. J. (2016). Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nature Communications, 7(1), 1–12. https://doi.org/10.1038/ncomms13010
Shichida, Y., & Matsuyama, T. (2009). Evolution of opsins and phototransduction. In Philosophical Transactions of the Royal Society B: Biological Sciences (Vol. 364, Issue 1531, pp. 2881–2895). Royal Society. https://doi.org/10.1098/rstb.2009.0051
Wheelwright, M., Whittle, C. R., & Riabinina, O. (2021). Olfactory systems across mosquito species. In Cell and Tissue Research (Vol. 383, Issue 1, pp. 75–90). Springer Science and Business Media Deutschland GmbH. https://doi.org/10.1007/s00441-020-03407-2




 (No Ratings Yet)
(No Ratings Yet)