Nuclear sponges in embryonic stem cells
Posted by kjchalut, on 17 June 2014
Once upon a time, physicists got curious about the cytoskeleton. They characterised the cytoskeleton – using tools of soft matter, statistical and polymer physics – as a mesoscale material whose physical properties govern its dynamics. They showed that the cytoskeleton is an interconnected scaffold that, depending on the time scale, can behave like a shape-morphing gel (slow) or like an elastic polymer network (fast)1. Concurrently, biochemists showed how molecules interact to mediate the architecture of this scaffold. Together, biochemists and physicists have illustrated an emergent picture of comprehensive cytoskeletal function; they enabled a fuller comprehension of, for example, cell migration2, maintenance of cell shape3, and morphogenesis4. Essentially, the two lines of research linking the molecular scale to the polymer scale in the cytoskeleton have amplified one another in an entirely co-dependent manner.
Interestingly, if the cytoskeleton exists solely to maintain cell integrity, and drive migration and molecular traffic flow, one would think cytoskeletal polymers would begin and end at the cellular surface. However, they do not: they are vitally connected, through nesprins, lamins and the LINC complex5,6, to the nucleus and the genetic machinery of the cell7. Why is the genetic machinery of the cell coupled to forces from the outside world? This is an especially vexing question to ask of embryonic stem (ES) cells, given that they lack the Lamin A/C protein8 that largely provides the nucleus its mechanical integrity9. Observe below the dramatic shape changes of the ES cell nucleus within one minute (click to play):
These shape changes are generated by the cytoskeleton. However, some might say the cell is devoted to a pre-ordained genetic program independent of the outside world modulo chemical signaling. Then why does the nucleus coordinate its shape with cytoskeletal dynamics? One potential explanation is mechanotransduction, which posits that forces from the outside world are transduced into changes in cell function. An idea within the mechanotransduction framework is that cytoskeleton-driven nuclear shape changes and subsequent rearrangements of chromatin precipitate changes in gene expression. It is now high time – in the days of CHiP and HiC highlighting chromosomal molecular interactions10,11 – for physicists to do for the nucleus what they have done for the cytoskeleton: provide a material foundation.
Against this backdrop, we set out to understand how ES cell nuclei respond to forces.
Specifically, we wished to know how nuclei of ES cells exiting pluripotency respond to forces. We investigated mouse ES cells maintained in a naïve pluripotent state using inhibitors described elsewhere12. By removing the inhibitors from the medium, ES cells begin to differentiate, and after approximately one day in transition, they exit pluripotency and prime for differentiation. We were especially interested in comparing these transition ES cells – the ones that are exiting the pluripotent state – to naïve pluripotent ES cells. Details of how we defined naïve and transition ES cells can be found in our recent work published in Nature Materials10.
We used an atomic force microscope (AFM) to compress naïve and transition ES cells. We found that the nuclei of transition ES cells, in contrast to naïve ES cells, shrank in cross-section upon compression. This counters expectations. If one presses, for instance, on a ball, couch cushion, or plastics, they would be surprised if these items shrank in cross-section when they press. Nevertheless, that’s what happens to the transition ES cell nucleus.
For further exploration, we tested what happens when tensile stresses are applied via the cytoskeleton. We developed a microfluidic system comprising channels smaller in cross-section than ES cells, but larger than average nuclei. The cytoplasm elongates, and applies tensile forces to the nucleus. Ordinarily, with applied tensile force a material, such as a rubber band, becomes thinner in cross-section and approximately conserves volume. This is what occurs with naïve nuclei, and nuclei of other cell types we investigated (including lineage primed cells). However, transition nuclei – against anticipation – expand in cross-section with applied tensile stress; indeed, the nuclear volume increases by up to 50%.
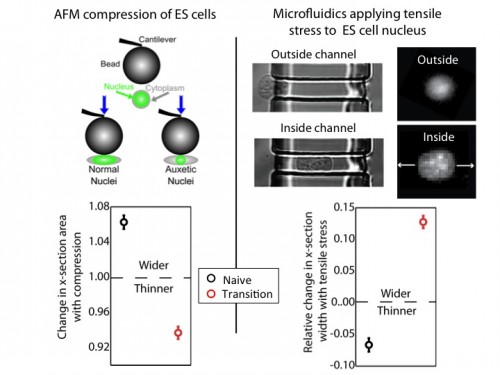
At left, we show that by compressing transition, but not naive, ES cells with AFM, we see that the nucleus shrinks. At right, we show that by using our microfluidic system to stretch cells and apply tensile forces to the nucleus, the nucleus gets wider in cross-section in transition ES cells, but not naive ES cells.
A material that expands in cross-section when stretched and shrinks in cross-section when compressed is called auxetic13. It is an unusual material property (one must imagine a rubber band getting fatter when stretched) not generally observed in biological materials. It is used for materials like bulletproof vests and soundproofing, due to its ability to immediately absorb impact energy throughout the material instead of just at the impact point. It is also a useful property for materials used for absorption – like sponges – due to the large volume gain and consequent opening of space resulting from a stretch (consider a sponge mop that is pulled, expanding potential fluid pockets in the material).
We investigated possible mechanisms for this unusual nuclear property. We looked for differences in nuclear envelope proteins such as Lamin B1 and Nesprins, but found no meaningful difference between transition and naïve ES cells. However, electron microscopy images showed that transition nuclei were more granular and less dense than naïve nuclei. Therefore, we induced chromatin decondensation using Trichostatin A (TSA)14. With TSA, we provoked an auxetic nucleus in naïve ES cells. This led us to conclude that auxetic nuclei are due, at least in part, to increased global chromatin decondensation in transition ES cells. This is a relatively surprising result from a biological perspective, given the conventional wisdom that naïve ES cells are in state of minimum global chromatin decondensation. However, given that auxetic materials tend to be low density, it was entirely predictable from the standpoint of materials science.
Finally, we addressed the potential function of an auxetic nucleus. We suspected that the function might lie in the dramatic absorptive properties implied by auxeticity. To explore this potential function, we first loaded both transition and naïve ES cells with fluorescein, which in cells becomes more highly concentrated in the cytoplasm than the nucleus. We used our microfluidic technique to apply tensile stresses to the nucleus, and observed fluorescein flowing from the cytoplasm into the nucleus in the transition ES cells, but not in the naïve ES cells. This result indicates that auxeticity opens up porous space in transition nuclei. Coming back to the possible function of an auxetic nucleus – and auxetic sponges – we now hypothesise that auxeticity may prove to be a mode of mechanotransduction. Tensile stresses would lower the free energy barrier associated with nuclear translocation, delivering differentiation specific signaling factors from the cytoplasm to the nucleus in the differentiating ES cell. This is an especially compelling idea given the transition of epiblast from a compressed epithelial phenotype to a spread mesenchymal phenotype after implantation, when they are primed for differentiation.
The idea of a nuclear sponge facilitating an increased differentiative capacity is an enticing prospect for the function of auxeticity, but there are other possibilities. We noticed with our AFM studies that transition ES cells (not naïve ES cells) stiffened with increased compression, as one would expect with an auxetic material. Possibly, this stress-driven stiffening (and conversely softening with tensile stress) could have a significant effect on dynamic sorting of nascent tissue layers within the developing embryo.
Ultimately, there is much work to be done to understand the how and why of auxetic nuclei in ES cells. It is, however, exemplary of new discoveries in biology made by applying physics to biological systems. We hope this discovery will be coupled to emerging awareness of molecular and mechanical signaling in the nucleus to generate a richer comprehension of pluripotency, differentiation, and embryogenesis.
1. Deng, L., Trepat, X., Butler, J., Millet, E., Morgan, K., Weitz, D., & Fredberg, J. (2006). Fast and slow dynamics of the cytoskeleton Nature Materials, 5 (8), 636-640 DOI: 10.1038/nmat1685
2. Gardel, M., Schneider, I., Aratyn-Schaus,, Y., & Waterman, C. (2010). Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration Annual Review of Cell and Developmental Biology, 26 (1), 315-333 DOI: 10.1146/annurev.cellbio.011209.122036
3. Paluch, E., & Heisenberg, C. (2009). Biology and Physics of Cell Shape Changes in Development Current Biology, 19 (17) DOI: 10.1016/j.cub.2009.07.029
4. Lecuit, T., Lenne, P., & Munro, E. (2011). Force Generation, Transmission, and Integration during Cell and Tissue Morphogenesis Annual Review of Cell and Developmental Biology, 27 (1), 157-184 DOI: 10.1146/annurev-cellbio-100109-104027
5. Lombardi, M., Jaalouk, D., Shanahan, C., Burke, B., Roux, K., & Lammerding, J. (2011). The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton Journal of Biological Chemistry, 286 (30), 26743-26753 DOI: 10.1074/jbc.M111.233700
6. Swift, J., Ivanovska, I., Buxboim, A., Harada, T., Dingal, P., Pinter, J., Pajerowski, J., Spinler, K., Shin, J., Tewari, M., Rehfeldt, F., Speicher, D., & Discher, D. (2013). Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation Science, 341 (6149), 1240104-1240104 DOI: 10.1126/science.1240104
7. Kind, J., Pagie, L., Ortabozkoyun, H., Boyle, S., de Vries, S., Janssen, H., Amendola, M., Nolen, L., Bickmore, W., & van Steensel, B. (2013). Single-Cell Dynamics of Genome-Nuclear Lamina Interactions Cell, 153 (1), 178-192 DOI: 10.1016/j.cell.2013.02.028
8. Pagliara, S., Franze, K., McClain, C., Wylde, G., Fisher, C., Franklin, R., Kabla, A., Keyser, U., & Chalut, K. (2014). Auxetic nuclei in embryonic stem cells exiting pluripotency Nature Materials, 13 (6), 638-644 DOI: 10.1038/nmat3943
9. Pajerowski, J., Dahl, K., Zhong, F., Sammak, P., & Discher, D. (2007). Physical plasticity of the nucleus in stem cell differentiation Proceedings of the National Academy of Sciences, 104 (40), 15619-15624 DOI: 10.1073/pnas.0702576104
10. Bickmore, W., & van Steensel, B. (2013). Genome Architecture: Domain Organization of Interphase Chromosomes Cell, 152 (6), 1270-1284 DOI: 10.1016/j.cell.2013.02.001
11. Nagano, T., Lubling, Y., Stevens, T., Schoenfelder, S., Yaffe, E., Dean, W., Laue, E., Tanay, A., & Fraser, P. (2013). Single-cell Hi-C reveals cell-to-cell variability in chromosome structure Nature, 502 (7469), 59-64 DOI: 10.1038/nature12593
12. Ying, Q., Wray, J., Nichols, J., Batlle-Morera, L., Doble, B., Woodgett, J., Cohen, P., & Smith, A. (2008). The ground state of embryonic stem cell self-renewal Nature, 453 (7194), 519-523 DOI: 10.1038/nature06968
13. Evans, K.E., & Alderson, A. (2000). Auxetic Materials: Functional Materials and Structures from Lateral Thinking! Advanced materials, 12 (9), 617-628
14. Chalut, K., Höpfler, M., Lautenschläger, F., Boyde, L., Chan, C., Ekpenyong, A., Martinez-Arias, A., & Guck, J. (2012). Chromatin Decondensation and Nuclear Softening Accompany Nanog Downregulation in Embryonic Stem Cells Biophysical Journal, 103 (10), 2060-2070 DOI: 10.1016/j.bpj.2012.10.015


 (2 votes)
(2 votes)