See below a posting from our website (http://amapress.gen.cam.ac.uk/) on the discussion that took place at the BSDB on whether to change or not the name of the society to include Stem Cell Biology. Some of the people who have read it have encouraged me to post it here to see what people think and I believe is a good idea (to open it up to discussion). In many ways it follows the spirit of Olivier Pourquie’s editorial at the beginning of the year in Development (http://dev.biologists.org/content/139/1/1.full)
With my wishes for a good Easter
Stem Cells in Developmental Biology: a debate at the BSDB
Last week the BSDB (British Society for Developmental Biology) celebrated its annual gathering at Warwick. Always a good place to go for quality developmental biology which is enhanced by the arrangement of holding the meeting together with the BSCB (British Society for Cell Biology): these days there is much cell biology in developmental biology. One of the BSDB sessions focused on Stem Cells and highlighted the clear connection between this area of research and developmental biology, or so it seems to some of us but ……perhaps not all.
The AGM of the BSDB had, for the second year running, a ballot to change the name of the society to include “stem cells’ in its name. Thus, the proposal was to change the name from “British Society of Developmental Biology” to “British Society of Developmental and Stem Cell Biology”. The proposal had been flagged last year and, after a vigorous discussion, was rejected, but by a narrow margin which allowed the subject to be brought up again to the AGM this year, where it was resoundingly rejected. But read on……….
The discussion preceding the vote was heated and highlighted several misconceptions about research in stem cells which, perhaps, represent some reality.
The ones in favour argued, correctly I think (I was and am in favour of the change) that Stem Cell research is part of developmental biology and that while there is much that has to do with medicine, the links between Developmental and Stem Cell Biology are strong and essential for both fields, that Developmental Biology can bring rigour and direction to Stem Cell Biology and that Stem Cell Biology can bring challenges, new ways and possibilities for Developmental Biology. They (we) argued that, after all, Stem Cell biology has always been part of Developmental Biology, albeit somewhat cryptically. Including Stem Cells in the name of the society is an acknowledgement of the times and can have its benefits because there is no denying that Stem Cell Biology is a central and key element of research these days; including it explicitly in the Societie’s agenda would allow the BSDB to have a strong voice in policy, funding and education in these increasingly influential area of research.
The ones against the change argued that Stem Cell Biology is different from Developmental Biology, that it has a clinical slant which would attract a different crowd to the meeting, force different content in sessions and, overall, distract us from the main business: the workings and evolution of embryos and systems. It was suggested that such a change would alienate several established constituencies within the society that would abandon the group. Mpre significantly, that, eventually, with another vote a few years down the line, Developmental Biology would be booted out of the title for the society to become the British Society for Stem Cell Biology.
As far as a I am concerned this was a missed opportunity. While I appreciate many of the points made by the ‘noes’ , I feel that their arguments are based on fear for a future that will always take over no matter what we do and that, in the long term, there will be consequences from not seeing this. We live in an increasingly corporate world in which lobbies are important and, in the context of our business, provide the basis for funding and policy. For the Society of Developmental Biology Society to have an explicit voice in the Stem Cell community is not just an extension of its natural remit and interests, but it is a way to bring some real science into a field that is increasingly interested in applications without having covered the bases. The foundations of Stem Cell Biology lie in Developmental Biology and it is important that developmental biologists have a say on decision making in that important field. Stem Cell Biology is not exactly, as some people claim, a new area of research (developmental biologists have been working with stem cells and their lineages for years) but it is certainly an area that recently has come of age to carve its own intellectual niche like, in many ways, Developmental Biology did in the 1970s (let us not forget that Developmental Biology is an offshoot of Embryology). It was argued that Stem Cells are born with Till and McCulloch (1964 Proc Nat Acad Sci. 51, 29–36). True but what they were looking at is the question of the origin of the blood, a problem in Developmental Biology whether one likes it or not. At certain places, and stem cell research is one of them, boundaries blur. Is genomics and bioinformatics genetics? Yes it is. In my book, Stem Cell Biology is part, and a very important part of Developmental Biology.
But let us move away from the heart of the question (of course the scientific content) and look, briefly, to the context of the discussion. The boundary, as I have said is blurred, and a situation can develop (and in certain places is happening) that some people, funding bodies, society, come to see Developmental Biology through the eyes of an unbridled Stem Cell Biology. After all, is it not organs out of cells that is the goal of stem cell biology? And is it not understanding these processes the goal of Developmental Biology? Then, what is the difference? The answer is simple, Stem Cell Biology wants to do, Developmental Biology wants to understand. It would be a pity not to bring them together. One can see here history trying to repeat itself: throughout the XVIII and XIX century engineers and inventors were making steam engines with little knowledge of physics, and they worked, but it is when the knowledge of thermodynamics is brought into the frameowork of the engineers that the engines become efficient. The same can be said of computing where, again, it is physics that makes the hardware that we have today. The fundamental science will always help the more applied side and needs it. So, much to be gained from Developmental Biology having a say in Stem Cell Biology. But there is a second more difficult question: what will be the consequences of the agenda of stem cells running that of developmental biology?
I can see a marginalization of model organisms and a biasing of the agenda towards applied science, applied in a trial and error way, rather than in the tradition of Science. I might be wrong in the extreme formulation of these concerns but I am certain that some of this will happen.
In the end, my impression was that the ‘noes’ were afraid, afraid of the future without realizing that he future will happen and that by not seeing the trends and joining them, we shall always be left to mercy of those trends, without a voice to influence them. I worry that model systems that have taught us so much about basic biology will slowly be squeezed into corners because we do not have a voice to explain that flies have stem cells, that stem cells are part of the make up of an organism which cannot be understood outside its context, that stem cells are a problem of evolutionary biology, that stem cells are a linguistic twist of linage analysis and lineage analysis has always been a problem of developmental biology, from Roux and Driesch to Garcia Bellido with Till and McCulloch in the middle. Incorporating Stem Cell Biology in the name of the society would have been a way of having a strong voice in a trend that is rapidly gaining momentum.
We shall see what the future harbours. The BSDB is a strong society which represents a vibrant and engaging community so there is no reason why things will change rapidly. However, one thing is clear: there is a need for the voice of developmental biologists to be heard in the Stem Cell community. A mechanism needs to be found for this. It is necessary as much to have a representation to remind that community where their real roots lie and the benefits of listening to the fundamentals of their field. There is a drift which was, unfortunately, at the heart of many of the speeches for the no, that Stem Cell Biology is more clinically that basic research orientated. One can see how this can be construed but, decisions like the one we have taken will increase this gap and foster this misunderstanding. It would be good (it is always good) to take lessons from history. As the interactions between physics and engineering prove, there is much to be gained from the interactions between a field that tries to find practical solutions and one that explain the causes of the problems. Let us hope that the BSDB can find a way to influence some of the directions of Stem Cell biology. For the moment it is as if two twins have been separated; each with their own mind but with shared genetics.
 (6 votes)
(6 votes)
 Loading...
Loading...
 Voting for the stem cell image contest opened earlier this month, and we’ve had close to 2000 votes in so far. It looks like a close race between a couple of the images – which is your favourite? Get your vote in if you haven’t done so already! Voting will close at noon GMT on Wednesday 10th April.
Voting for the stem cell image contest opened earlier this month, and we’ve had close to 2000 votes in so far. It looks like a close race between a couple of the images – which is your favourite? Get your vote in if you haven’t done so already! Voting will close at noon GMT on Wednesday 10th April. – Over the last couple of years, Kim Cooper (a post-doc in the Tabin lab) has been providing us with updates (see her intro post here) from her multiple trips to China, during which time she has been collecting jerboas. This month, Kim shared with us the news of the first research article using jerboas to answer a fundamental question in developmental biology.
– Over the last couple of years, Kim Cooper (a post-doc in the Tabin lab) has been providing us with updates (see her intro post here) from her multiple trips to China, during which time she has been collecting jerboas. This month, Kim shared with us the news of the first research article using jerboas to answer a fundamental question in developmental biology.

 (No Ratings Yet)
(No Ratings Yet)
 (6 votes)
(6 votes) An unproven stem cell therapy has taken centre stage in Italy after patients successfully lobbied the Italian government to allow its use in public hospitals. The highly controversial and untested procedure was created by the privately owned Stamina Foundation, but blocked by the Italian Medicine’s Agency, AIFA. Last week’s decision by the Ministry of Health to override AIFA’s block has horrified Italy’s leading stem cell scientists. In a letter to the Ministry, they describe the decision as providing “a dangerous short circuit between patients’ hopes and lucrative commercial practices” of organisations operating outside the “scientific and moral foundations” of medicine.
An unproven stem cell therapy has taken centre stage in Italy after patients successfully lobbied the Italian government to allow its use in public hospitals. The highly controversial and untested procedure was created by the privately owned Stamina Foundation, but blocked by the Italian Medicine’s Agency, AIFA. Last week’s decision by the Ministry of Health to override AIFA’s block has horrified Italy’s leading stem cell scientists. In a letter to the Ministry, they describe the decision as providing “a dangerous short circuit between patients’ hopes and lucrative commercial practices” of organisations operating outside the “scientific and moral foundations” of medicine.  Our
Our 
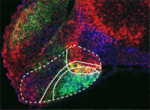 The hypothalamus is a key integrative centre in the vertebrate brain that regulates many essential functions, including homeostasis and stress responses. Several transcription factors that are essential for hypothalamic development have been identified but the production of diverse neuron types in this complex brain region is poorly understood. Here (
The hypothalamus is a key integrative centre in the vertebrate brain that regulates many essential functions, including homeostasis and stress responses. Several transcription factors that are essential for hypothalamic development have been identified but the production of diverse neuron types in this complex brain region is poorly understood. Here (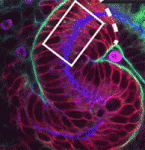 The formation and elongation of polarised epithelial tubules is essential for the structure and function of several metazoan organ systems but the molecular mechanisms that regulate tubulogenesis are largely unknown. Here (
The formation and elongation of polarised epithelial tubules is essential for the structure and function of several metazoan organ systems but the molecular mechanisms that regulate tubulogenesis are largely unknown. Here ( Planar cell polarity depends on the asymmetric localisation of core planar polarity proteins at apicolateral junctions. This asymmetric distribution probably develops through amplification of an initial asymmetry and seems to require the regulation of core protein levels. Now, Helen Strutt, Elizabeth Searle and co-workers (
Planar cell polarity depends on the asymmetric localisation of core planar polarity proteins at apicolateral junctions. This asymmetric distribution probably develops through amplification of an initial asymmetry and seems to require the regulation of core protein levels. Now, Helen Strutt, Elizabeth Searle and co-workers (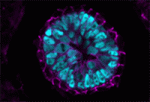 During mammalian embryogenesis, substantial cell proliferation occurs before the establishment of the body plan during gastrulation. Thus, before gastrulation, individual embryonic cells must be pluripotent. In vitro experiments with embryonic stem cells (ESCs) have indicated that the transcription factors Oct4, Sox2 and Nanog are components of a gene regulatory network (GRN) that stimulates self-renewal of pluripotent cells and have identified Tcf7l1 (Tcf3) as an inhibitor of GRN activity. But what is Tcf7l1’s function during embryonic development? To find out, Bradley Merrill and colleagues have been examining embryogenesis in Tcf7l1-/- mouse embryos (see
During mammalian embryogenesis, substantial cell proliferation occurs before the establishment of the body plan during gastrulation. Thus, before gastrulation, individual embryonic cells must be pluripotent. In vitro experiments with embryonic stem cells (ESCs) have indicated that the transcription factors Oct4, Sox2 and Nanog are components of a gene regulatory network (GRN) that stimulates self-renewal of pluripotent cells and have identified Tcf7l1 (Tcf3) as an inhibitor of GRN activity. But what is Tcf7l1’s function during embryonic development? To find out, Bradley Merrill and colleagues have been examining embryogenesis in Tcf7l1-/- mouse embryos (see 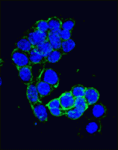 Canonical Wnt signalling and E-cadherin-mediated cell adhesion are both involved in mouse embryonic stem (mES) cell maintenance. β-catenin (Ctnnb1) is central to both these processes – it mediates the transactivation of Wnt target genes and also connects E-cadherin to the actin cytoskeleton via α-catenin. But which β-catenin function is absolutely required for mES cell self-renewal and pluripotency? On
Canonical Wnt signalling and E-cadherin-mediated cell adhesion are both involved in mouse embryonic stem (mES) cell maintenance. β-catenin (Ctnnb1) is central to both these processes – it mediates the transactivation of Wnt target genes and also connects E-cadherin to the actin cytoskeleton via α-catenin. But which β-catenin function is absolutely required for mES cell self-renewal and pluripotency? On 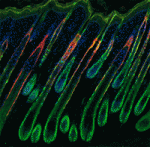 Adult tissue-specific stem cells both self-renew and generate functional progeny. Mammalian hair follicles, which are characterised by cyclical phases of growth (anagen), regression (catagen) and rest (telogen), are an ideal system in which to investigate the homeostasis of an adult stem cell population. On
Adult tissue-specific stem cells both self-renew and generate functional progeny. Mammalian hair follicles, which are characterised by cyclical phases of growth (anagen), regression (catagen) and rest (telogen), are an ideal system in which to investigate the homeostasis of an adult stem cell population. On 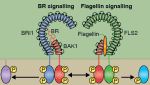 Zhi-Yong Wang and colleagues provide an overview of the highly integrated BR signalling network and explain how this steroid hormone functions as a master regulator of plant growth, development and metabolism.
Zhi-Yong Wang and colleagues provide an overview of the highly integrated BR signalling network and explain how this steroid hormone functions as a master regulator of plant growth, development and metabolism.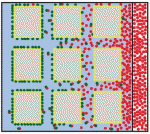 Alex Schier et al analyze various morphogen transport models using the morphogens Nodal, fibroblast growth factor and Decapentaplegic as case studies. They propose that most of the available data support the idea that morphogen gradients form by diffusion that is hindered by tortuosity and binding to extracellular molecules.
Alex Schier et al analyze various morphogen transport models using the morphogens Nodal, fibroblast growth factor and Decapentaplegic as case studies. They propose that most of the available data support the idea that morphogen gradients form by diffusion that is hindered by tortuosity and binding to extracellular molecules. This workshop is geared toward university faculty, postdocs, and graduate students interested in teaching developmental biology. It provides basic hands-on experience with organisms commonly studied in teaching laboratories. These include sea urchins and sand dollars, planaria, Drosophila, chick embryos, Spirostomum, Hydra, Lumbriculus, and flowering plants. Techniques will range from classical microsurgical techniques to fluorescence microscopy and applications of reporter gene technology.
This workshop is geared toward university faculty, postdocs, and graduate students interested in teaching developmental biology. It provides basic hands-on experience with organisms commonly studied in teaching laboratories. These include sea urchins and sand dollars, planaria, Drosophila, chick embryos, Spirostomum, Hydra, Lumbriculus, and flowering plants. Techniques will range from classical microsurgical techniques to fluorescence microscopy and applications of reporter gene technology.
 (8 votes)
(8 votes)