The people behind the papers – Ivette Olivares-Castiñeira & Marta Llimargas
Posted by the Node Interviews, on 21 July 2017
The Drosophila tracheal system is a powerful model for understanding the genetic and cell biological control of tubulogenesis. In their new PLoS Genetics paper, Ivette Olivares-Castiñeira and her PI Marta Llimargas of the Molecular Biology Institute of Barcelona connect EGFR signalling to intracellular cell trafficking during tracheal morphogenesis. We caught up with Marta and Ivette to hear the story behind the paper.

Marta, can you give us your scientific biography and the main questions your lab is trying to answer?
MA I studied Biology at the University of Barcelona where I took a course on developmental biology and embryology, given by Dr. Jaume Baguña that made me enthusiastic about development. Then I was very lucky because Dr. Jordi Casanova, a well-known Drosophila developmental biologist, just got a position in Barcelona and he accepted me as a PhD student in his lab. By chance we started to work on tracheal development, and I became fascinated by the development of such a beautiful and interesting tissue. The analysis of tracheal development was just an emerging field by then and this allowed me to follow and contribute to the field during all these years.
In 1997 I moved for a postdoctoral stay in Dr. Peter Lawrence’s lab, at the LMB-MRC in Cambridge. There I had an incredible scientific and personal experience, and I was encouraged by Peter to develop my own projects. Because I was really thrilled by tracheal development and Peter allowed me the freedom, I continued working on the subject while there. In 2002 I moved back to Barcelona where I started my lab and later, in 2007, I got a permanent position at the Molecular Biology Institute of Barcelona. My lab has always been, and still is, interested in tracheal formation as a model to understand the morphogenesis of branched tubular organs. We are interested in the genetic mechanisms that drive tracheal formation and in the molecular mechanisms used by these genetic networks to instruct the cellular changes that underlie organ formation.
With the recent announcement of a new EMBL site and a number of universities and research institutes, Barcelona seems to be an exciting place for life sciences at the moment?
MA Yes, Barcelona has become a real hub for science in the past few years and at the moment it is an excellent place for researchers. Barcelona is attracting many senior and young researchers, from many different areas of research. This provides an exciting and encouraging atmosphere for interdisciplinary research. Moreover, many pharmaceutical and biotech companies are set in Barcelona, which also encourages translational research and transfer of knowledge. Obviously the lifestyle, the climate and being a medium sized city also positively contribute to the success of Barcelona as a scientific hotspot.
Barcelona has a number of very good institutions and the production of high quality science in the region is high. Some institutions are very good and very visible, but there are other good institutions and talented people that are less known and overall people struggle with lack of consistent funding to properly develop their research. The general funding situation for science is still below what would be required to fully take advantage of the people and resources.
And Ivette, how did you come to join the Llimargas lab?
IO-C I joined Marta’s lab in 2013, when I had just finished my master’s degree and I realised that I would like to do a PhD. I was looking for an opportunity and Marta gave me the chance to join her lab. After the first year, I became interested in analysing how EGFR was controlling the development of the tracheal system. Being in a developmental biology lab gave me the opportunity to investigate the mechanisms of organ and tissue formation. I find Drosophila tracheal development particularly interesting and ideal to analyse the genetic control of morphogenesis and the cellular mechanisms at play. Doing my PhD with Marta is a great experience for my future career and allows me to get insights into the morphogenesis of epithelial tissues.
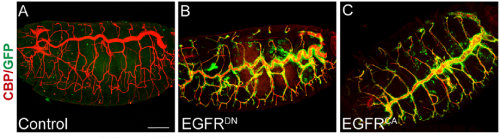
What was known about the role of EGFR signalling in tracheal development before your paper?
MA & IO-C We already knew a few things. Our lab had published work on the role of EGFR during embryonic tracheal development, in which we showed that it is required for invagination of tracheal cells and to maintain epithelial integrity. In addition, other labs, like Dr. Casanova’s and Dr. Hayashi’s, had also investigated the role of EGFR in tracheal invagination and identified an EGFR-dependent regulation of Myosin-II.
However, a role for EGFR in tube growth was not previously reported. It was actually, back in 2006, during our analysis of EGFR signalling on epithelial integrity that we noticed the effect of EGFR on tube length. However, this observation coincided with the end of the PhD of the student working on this project and it was not followed at the time. When Ivette started her PhD we thought that investigating EGFR requirement on tracheal elongation was a good project to explore, also because there was much more information about tracheal tube growth at the moment and we felt it was the right time to approach the issue. In addition we were also interested in analysing in more detail the possible different molecular mechanisms of EGFR underlying the different tracheal requirements such as invagination, integrity and tube length mainly at the cell biology level.
After analysing EGFR activity in our and other labs, we now know that, as it happens in many other organs and tissues in Drosophila and in other organisms, EGFR is used reiteratively during tracheal formation, but the exact molecular mechanisms behind each of these requirements still need to be fully clarified.
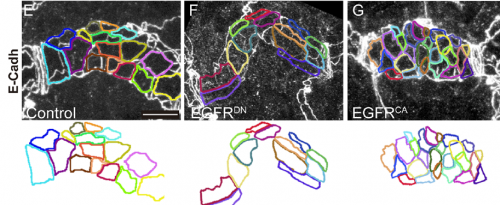
Can you give us key results of the paper in a paragraph?
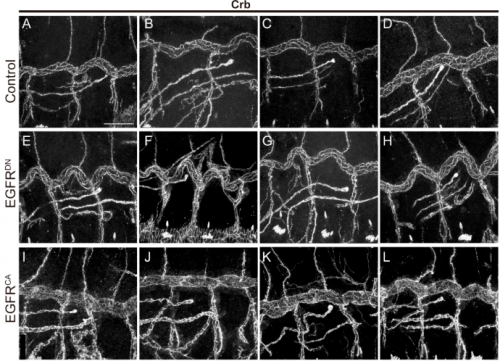
MA & IO-C We have found that EGFR is required to prevent an excessive growth of the tracheal tubes. EGFR is required for the proper accumulation and localisation of two previously known regulators of tube length, the apical determinant Crumbs and the apical extracellular matrix (aECM) regulator Serpentine. This positions EGFR as a hub coordinating cell intrinsic properties (Crumbs-mediated apical expansion) and cell-extrinsic mechanisms (Serpentine-mediated modification of the aECM) regulating tube length. Interestingly we found that these two proteins accumulate in common sorting endosomes, and that EGFR is required for the proper organisation of these common endosomes, likely regulating the correct delivery of both cargoes to their final destination. We observed that the two cargoes are partitioned into different discrete domains within this common sorting endosome, consistent with the hypothesis that they use different retrieval pathways to recycle. We also observed that, during tracheal development, Crumbs undergoes a complex pattern of recycling, which involves internalisation and different sorting pathways. Our results illustrate a role for EGFR in endocytic trafficking, a molecular mechanism that could potentially underlie different developmental and pathogenic EGFR activities.
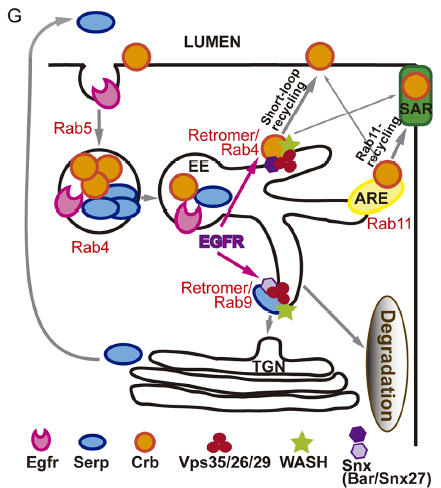
What do you think the EGFR receptor is doing in the endosomes?
MA / IO-C That is what we would like to unravel! Many reports in the literature describe the internalisation and trafficking of EGFR receptor, the many factors involved in the process and the consequences for EGFR activity. So obviously EGFR is a cargo of endocytic trafficking, and this trafficking regulates its signal termination and the recycling of the receptor to the membrane. Much less is known about a possible role of the receptor itself in controlling its own or other protein’s trafficking. Our results point to a role of EGFR in organising the endosome, because when EGFR is downregulated endosomes are bigger, cargoes are mis-sorted and WASH accumulation, which is recruited by the Retromer complex, is also affected. Whether this is a direct or an indirect effect of EGFR downregulation is still unclear. However, the fact that we find EGFR itself in Crumbs/Serp sorting endosomes and the fact that endosomes are affected leads us to speculate that EGFR regulates targets in the endosome. In fact, EGFR cytoplasmic targets and endosomal targets have been identified, suggesting that intracellular organelles can act as EGFR signalling platforms. To get further insights into the molecular mechanism of EGFR in tube elongation we are working with the hypothesis that EGFR (or EGFR downstream effectors) signals from the endosome to organise this organelle.
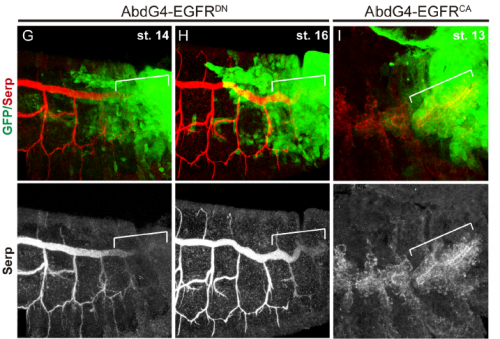
Do you have any clues as to how the apical ECM instructs the underlying tracheal epithelium during tube elongation?
MA / IO-C This is an outstanding question in the field. It is clear that the aECM and the underlying tracheal cells cross-talk and that this cross-talk is required for the correct tube growth in length and diameter. But the exact molecular mechanism is still unclear. The aECM can potentially instruct tracheal cells by exerting a mechanical force that is received by mechanosensors in the tracheal cells. It could also trap or expose chemical signal/s that could be received by tracheal cells. Or it could both act as a mechanical and a signalling platform. Bo Dong and Shigeo Hayashi have put forward a model where the apical membrane expansion force in tracheal cells that drives tube elongation is balanced with the resistance of an elastic aECM that restricts overelongation. The aECM mechanical tension and the apical expansion force are coupled through proteins attaching or coordinating the apical membrane to the aECM, such as Zona Pellucida proteins (as proposed by Hayashi lab) or Src42A (as proposed by Sofia Araújo and Jordi Casanova). Hopefully the dedicated work of several labs in the subject will soon shed light into this issue and we will better understand not only the growth of tubular structures but also how the cross-talk of extracellular matrices and organ/tissue/cells instructs morphogenesis.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
IO-C From the beginning of the work, our main goal was to identify the target/s of EGFR that control tracheal tube length. We found that Crumbs and Serpentine were affected in the tracheal tubes upon modulation of the EGFR activity. My eureka moment was when one afternoon, we realized that these two protein seemed to be more related than expected and we observed that they were loaded in common vesicles (we did not know that these were endosomes at the moment). It was a moment of euphoria as this was shedding light into a complicated puzzle of many pieces, although this also raised many other questions.
And what about the flipside: any moments of frustration or despair?
IO-C Science always has moments of frustration. It was particularly frustrating to repeat many times some of the stainings before we got good samples. In general, I find that technical details can often be really time-consuming and despairing. It was also frustrating to use several RNAi or dominant negative lines that did not produce phenotype, although I knew that the RNAi technique does not always work well for embryonic development. And finally, I found it very frustrating when we had different results but we were not able to understand what was happening. It is not always easy to put the pieces of the puzzle in the right place to get the final picture.
What are your career plans following this work?
IO-C First, I need to finish my PhD, which I plan to do this year. In the lab I am trying to get more details on the molecular mechanisms of EGFR and on the role of Crumbs in tube size. I am particularly interested in the connection of these proteins with intracellular trafficking pathways. After I finish my PhD, I will probably continue in the research world, as I find it fascinating.
And what next for the Llimargas lab?
MA Well, we are continuing different aspects derived from this work. For instance, we are now very interested in understanding Crumbs localisation in different apical subcellular domains (in trachea and other tissues) and whether this correlates with different Crumbs activities. Obviously this aspect is not only relevant for tracheal development, but may help to better understand the complexities of Crumbs. We are also interested in understanding how Crumbs, Serpentine, Vermiform, Src, EGFR and Dumpy, all of them known regulators of tube length, interact and regulate each other. Furthermore, we are really curious to understand how the retromer complex selects the different cargoes, Crumbs and Serpentine in this case, and sorts them into different recycling pathways. For this particular question we have identified a putative regulator of retromer trafficking, a nexin, that could provide cargo and itinerary specificity for the specific sorting of Serpentine and Crumbs. We are now investigating this aspect, generating mutants for this nexin and characterising its role. But obviously, the main question we are trying to answer is how EGFR regulates endocytic trafficking.
Besides this project, in the lab we are also interested in other aspects of tracheal formation, related with chitin deposition and with adhesion and polarity maintenance and remodelling.
Finally, what do you like to do when you are not in the lab?
MA I spend as much time as I can with my family. I try to get some time to read, to go to the theatre, to dance and to go out with friends. I love cooking for friends and family and I love eating, particularly new and surprising foods. I also exercise, particularly gym and running, in the nature when possible.
IO-C I try to switch off my mind from the lab issues and I like to go for run and do sport, which is good to clear my mind. Also, I like to read or to be with my friends. If I had more time I would like to travel more.
Ivette Olivares-Castiñeira, Marta Llimargas. 2017. EGFR controls Drosophila tracheal tube elongation by intracellular trafficking regulation. PLoS Genetics 1006882.
This is the 24th episode of our People Behind the Papers series – browse the archive here.


 (2 votes)
(2 votes)