The people behind the papers: Lijun Chi and Paul Delgado-Olguin
Posted by the Node Interviews, on 9 June 2017
Development of the placental vasculature – known as the labyrinth – is critical for foetal development. Today’s paper comes from the most recent issue of Development and addresses the signalling events involved in placental vascular maturation. We caught up with lead author Lijun Chi and her PI Paul Delgado-Olguin of the Hospital for Sick Children and University of Toronto.

Can you give us your scientific biography and the main questions your lab is trying to answer?
PD-O As a Ph.D. student at the Universidad Nacional Autónoma de México (UNAM) under supervision of Dr. Ramón Coral Vázquez and Dr. Félix Recillas Targa, I investigated the transcriptional regulation of genes expressed in skeletal muscle and whose mutations cause muscular dystrophies. My reading during my Ph.D. studies led me to numerous landmark papers from Eric Olson’s lab on skeletal muscle gene regulation. I soon discovered Dr. Olson’s and Dr. Deepak Srivastava’s seminal work on the transcriptional pathways controlling heart development, a process that I always found fascinating. Working at Dr. Recilla’s lab, I became very interested in the function of chromatin modifiers in gene control, which led me to mine the scientific literature to learn about the function of chromatin structure regulators in cardiac gene control and development. This made me realize how little was known on the subject at the time, and brought me across a pioneering paper from Dr. Benoit Bruneau’s lab describing an essential function of Baf60c, a subunit the SWI/SNF chromatin remodelling complex, in heart development. This helped me decide what I wanted to do as a postdoc.
I obtained my Ph.D. degree in June 2005, and by August I was in Dr. Bruneau’s laboratory ready to start my postdoctoral research. In Dr. Bruneau’s lab I investigated the function of the histone methyltransferase Ezh2 in the heart. This enzyme tri-methylates the lysine 27 of histone H3 to repress gene expression, and its global deletion causes lethality early in mouse development. Surprisingly, we found that deletion of Ezh2 in cardiac progenitor cells, despite altering embryonic gene expression, did not alter heart development, and mutant mice had normally structured hearts. However, adult mutants developed heart disease. This raised the possibility that epigenetic alterations in differentiating cardiovascular progenitor cells early in development might program adult heart disease susceptibility. To address this possibility, my lab at The Hospital for Sick Children (SickKids) has been studying the function of several histone modifiers in cardiac and vascular development, and in regulating adult cardiovascular system function since 2012. Because the placental vasculature is required for proper embryo development, and its malfunction can affect heart development and program adult disease in the offspring, my lab is also interested in uncovering the mechanisms controlling its development. My lab will continue to focus on uncovering the mechanisms controlling cardiovascular development and programming postnatal disease during embryogenesis.

And what is Toronto like to do science in?
PD-O Toronto is a great place to do science. It is home for numerous world-class scientists, research institutes, numerous hospitals with very strong research programs, and the University of Toronto, which has an outstanding research curriculum. These attributes make Toronto and ideal place to develop multidisciplinary research of the highest quality. For example, my lab investigates fundamental biological processes, and being at SickKids and in Toronto’s rich scientific environment allowed establishment of key collaboration with clinician scientists in neighbouring institutions, which has facilitated me to explore the translation potential of my lab’s research.
Lijun, how did you come to join the Delgado-Olguin lab?
I obtained my PhD. at the University of Oulu, Finland under the supervision of Professor Seppo Vainio. My PhD thesis, published in 2007, describes the role of Sprouty2 in development of the urogenital system. I then pursued a postdoctoral fellowship in Dr. Norman Rosenblum’s laboratory at SickKids in Toronto, Canada. During my fellowship I investigated the function of the cilia protein Kif3a, whose deficiency causes polycystic kidney in human and mouse models. After finishing my fellowship in 2011, I wanted to learn about cardiovascular development, and I knew Paul was just about to Join SickKids. Working at the Delgado-Olguin lab has given me the opportunity to work in exciting projects to understand the basis of cardiovascular development and disease.
What was known about signalling pathways controlling placental vascular maturation before you started this work?
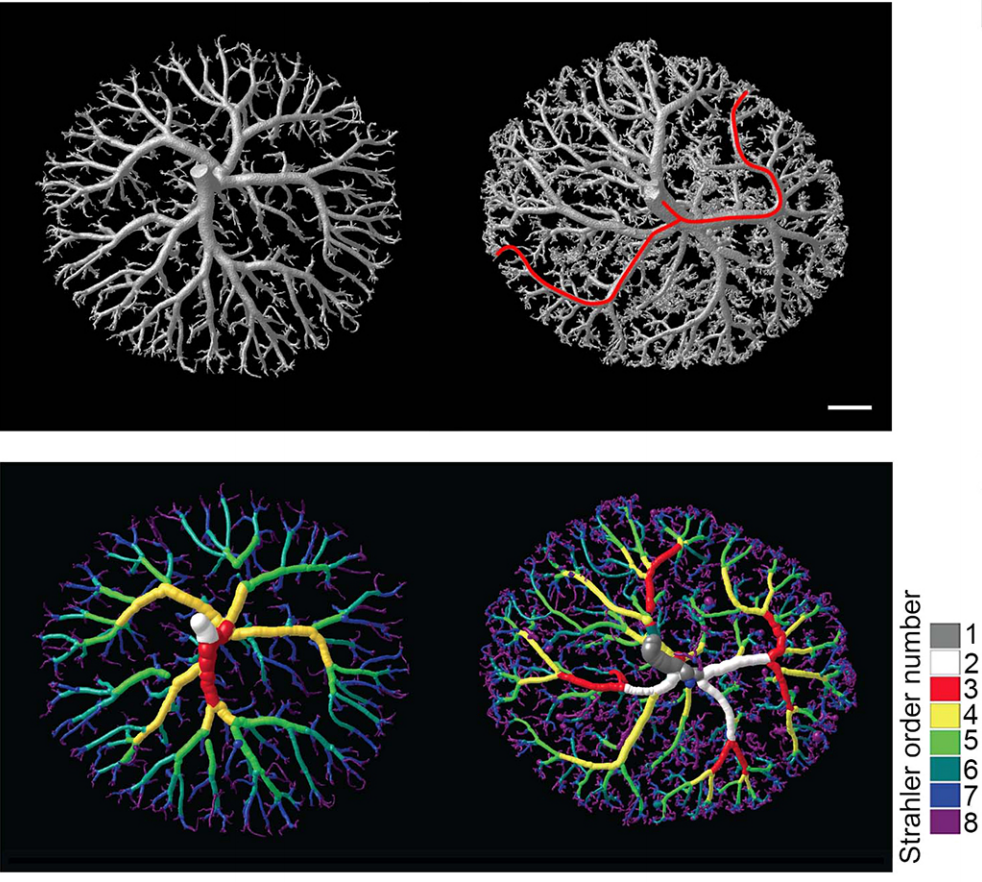
PD-O Because of its relevance in embryogenesis and in postnatal health, I was surprised to find out how little we knew about pathways controlling placental vascular maturation before we started our work. Perhaps the most informative report on the subject is from Knox & Barker (2008), who performed global gene expression analyses on the embryonic portion of mice placental vasculature, known as the labyrinth, at consecutive days of development. This analysis revealed a sharp molecular transition defining the developmental and the maturation phases of the placenta. In this transition, over 700 genes change their expression from embryonic day 12.0 to E13.5, and functions associated with these genes provided a general idea of some of the processes occurring in each phase. For instance, genes that are expressed in the developmental phase are involved in growth, metabolic processes, DNA and RNA processing, and cell cycle regulation. While genes active in the maturation phase are involved in pregnancy and reproduction. However, these studies were done on whole labyrinth, and thus tell us little on the pathways controlling this transition in specific cell types. To the best of my knowledge, our work is the first one to address the regulation of the transition from development to maturation in placental vascular maturation, and to define pathways active in endothelial cells regulating this process.
Can you give us key results of the paper in a paragraph?
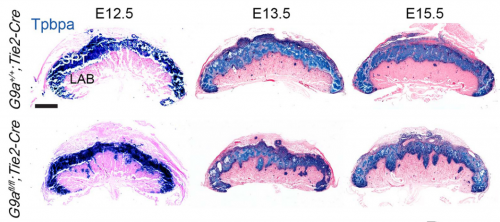
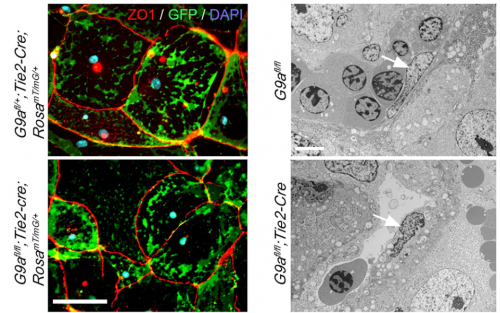
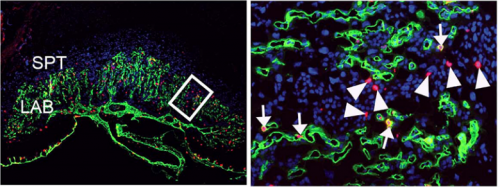
PD-O We found that the histone methyl transferase G9a activates the Notch pathway in endothelial cells to promote maturation of the placental vasculature. We inactivated the histone methyltransferase G9a in endothelial progenitors and their derivatives, and found that mutant embryos died with placental defects. Closer examination revealed that the gross morphology of the placentae from mutant embryos appeared normal at E12.5, but had a smaller vascularized area at E13.5. Because the transition from the developmental to the maturation phase of the placenta occurs precisely between these stages, this raised a possible function for G9a as a regulator placental vascular maturation. Analysis of cell proliferation revealed that growth of the labyrinth is coordinated with decreased growth of the spongiotrophoblast during the development to maturation transition, and that this balance is lost in G9a mutant placentae. To uncover regulatory pathways we performed global gene expression analysis, which revealed that effectors of the Notch pathway were downregulated in G9a mutant placental endothelial cells. We then introduced a transgene to activate the Notch pathway in G9a mutants, and we found that the placental morphology was rescued! This opened the possibility that a G9a-Notch axis might be disrupted in placental diseases with vascular maturation defects. Indeed, we found that G9a, and Notch regulators were downregulated in human placentae from pregnancies affected with intra uterine growth restriction.
How might your work inform efforts to diagnose or even treat placental defects during pregnancy?
PD-O These are very exciting possibilities. Intra uterine growth abnormalities are diagnosed only when the foetus is smaller than expected for the gestational age or when the placenta is already malfunctioning. The ability to identify preclinical placental insufficiency is limited because we know very little about the regulation of placental vascular development, and on the events that precede placental malfunction. We found that imbalanced growth of the labyrinth vs the spongiotrophoblast precedes the appearance of gross morphological abnormalities in G9a mutant placentae. Based on these results, we think that being able to define the growth ratio of these placental cell types, and detect imbalances might offer a way of identifying foetuses at risk of defective intrauterine growth. In terms of prevention or treatment, our findings open the possibility that activating the Notch pathway might be investigated as a means to promote placental vascular maturation. Our mouse model, combined with availability of pharmacologic compounds that activate the Notch pathway, will allow us to further investigate these possibilities.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
LC In the initial stage of the research, we were mainly focused on identifying cardiac defects in G9a mutants. However, I noticed that mutants had placentae with reduced vascularization. I decided to analyze embryos at consecutive developmental stages and I found that the vascular defect was obvious only at E13.5 and onwards. When I realized that the transition from the developmental to the maturation phase occurs precisely from E12.5 to E13.5, I hypothesized that G9a might regulate this transition. Given that the regulation of placental vascular maturation is very poorly understood, we decided to investigate further and test the hypothesis.
What about the flipside: any moments of frustration or despair?
LC As often happens with genome wide gene expression data, it was difficult to identify potentially relevant targets downstream of G9a from our RNAseq results. Fortunately, we found published reports demonstrating the involvement of the Notch signalling pathway in vascular maturation in the retina. We also found a report showing downregulation of some Notch regulators in placentae from pregnancies affected by intrauterine growth restriction. These reports encouraged me to confirm downregulation of Notch effectors in G9a mutant placental endothelial cells, and later on to test the effect of activating the Notch pathway in G9a mutant endothelial cells. These experiments were nerve wracking, because if the vascular phenotype were not to be corrected at least partially, I would have had to keep trying to identify functionally relevant G9a targets.
What are your career plans following this work?
LC With the completion of this current study, I am planning to test whether pharmacologically activating the Notch pathway in the G9a mutant placental vasculature promotes the maturation process and ameliorates or prevents placental defects. This might open the door to new experiments to try to promote vascular maturation in other models of intrauterine growth restriction.
And what next for the Delgado-Olguin lab?
PD-O We will delve deeper into the mechanisms by which G9a controls placental vascular maturation and the effects of activating Notch signalling in the placental vasculature. There are outstanding questions from our work. Particularly intriguing is how G9a activates the Notch pathway, as it is predominantly known as a transcriptional repressor, while its function as a transcriptional activator is less understood. Dissecting the mechanisms of action of G9a in placental endothelium will likely reveal additional regulatory pathways and potential approaches to promote vascular maturation. More broadly, our lab will continue to investigate the mechanisms by which postnatal cardiovascular disease is programmed during embryogenesis.
Finally, what do you two like to do in Toronto when you are not in the lab?
PD-O I enjoy hiking the many trails and parks in the city with my family and dogs, visiting museums, and fishing. Also, being an avid foodie, living in a city where there is food from all over the world available close by is a great bonus!
LC During my spare time, I am a passionate reader who enjoys a diversity of novels. I am also a skilful cook who loves exploring different recipes and trying out new styles. To stay physically well rounded, swimming is one of my weekly activities.


 (5 votes)
(5 votes)