Preventing cellular mixing with programmed cell death
Posted by Mikawa Lab, on 12 February 2020
By Lisandro Maya-Ramos and Takashi Mikawa
Bilaterality, the property of having two symmetrical sides, is widely conserved among animals. It is estimated that 99% of all animal species are bilaterians, with the remaining 1% composed by sponges and radial animals, which lack or have radial symmetry respectively (1). Although bilaterality is widespread among animals, little is known about how it is developmental patterned or shaped.
Observations from naturally occurring gynandromorphs such as birds, lobsters and butterflies (to name a few) suggest that cells from left and right sides remain on their own (ipsilateral) side, with little mixing seen through out life (2). In these animals the right and left sides, which have different phenotypic colors, meet at the body midline without crossing to the contralateral side. In fact, a similar phenomenon has been reported in humans: in a clinical case-report, a patient was found to have an ovary on the left side and a testicle on the right side, and further karyotyping of skin fibroblasts revealed XX sex chromosomes on left side and XY on the right (3). This information hints at the presence of mechanisms ensuring the patterning of ipsilaterality during development.
In the case of amniotes, this is particularly intriguing given that in early body patterning (gastrulation), cells undergo epithelial to mesenchymal transition (EMT) and these cells are known to be highly invasive (4). Therefore in the recent publication Maya-Ramos and Mikawa (5), we addressed the question of how is ipsilaterality patterned during amniote gastrulation. This question was best addressed using the chick embryo, given its long-standing history as a model system in gastrulation, its handling, easy accessibility, high temporal and spatial transfection control parameters and live imaging robustness.
Our results demonstrated that ipsilaterality is patterned during gastrulation; that is, right epiblast cells undergoing EMT gave rise to right mesoendodermal cells while left epiblast cells resulted in left mesoendodermal cells. Epiblast cells undergoing EMT seldom crossed the embryonic midline. These findings are consistent with the observations of bilateral gynandromorphs and human clinical case reports, and argue that left and right sides in bilaterians are established early in development.
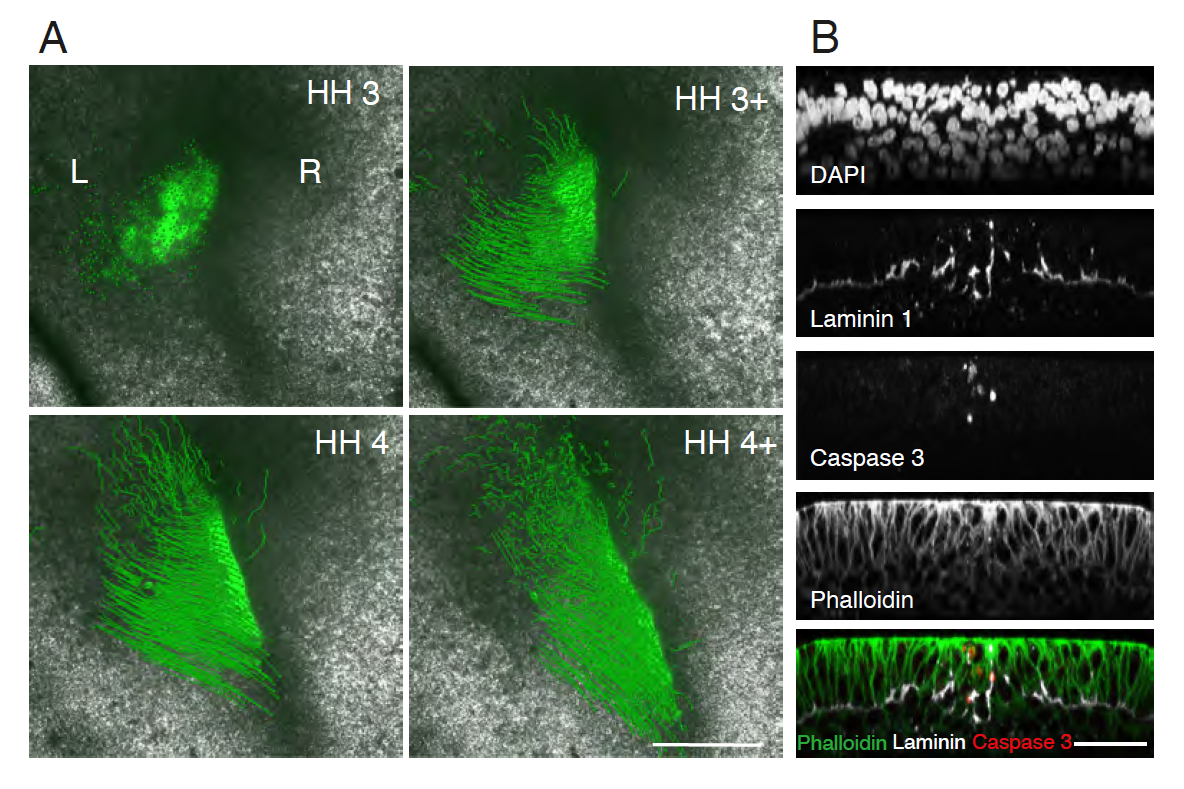
We found that the mechanism preventing cellular mixing was at the primitive streak (PS) midline. The PS midline was cellularly and molecularly distinct from PS lateral cells, as it was enriched with both extracellular matrix (ECM) proteins and programmed cell death (PCD). The origin of PS midline cells undergoing PCD was traced to a unique posterior embryonic region, embedded within the early PS. ECM and PCD loss of function resulted in crossing of the embryonic midline to the contralateral side. However, ipsilateral gastrulation was only restored with exogenous PCD.
These results highlight two key points. One is that PCD serves as a signal that prevents cell migration – this gives PCD a positive functional role in development. It is still unclear, however, what is the mechanism by which PCD prevents contralateral migration, for instance whether steps leading to PCD or the persisting cellular debris is responsible for this phenotype. Lingering cellular debris leading to intracellular content release has being associated with pathological processes, including Alzheimer’s disease, Parkinson’s disease and Systemic Lupus Erythematosus (6-8). Therefore, is not inconceivable that these same signals may take on a physiological role in development.
Second, these results suggest that ipsilaterality is programmed within bilaterality and that upstream signals are in place to specify PS midline cells before they undergo PCD. Therefore a persisting question is, how is the midline defined?
References
- M. Q. Martindale, J. R. Finnerty, J. Q. Henry, The Radiata and the evolutionary origins of the bilaterian body plan. Mol Phylogenet Evol 24, 358-365 (2002).
- S. Aw, M. Levin, What’s left in asymmetry? Dev Dyn 237, 3453-3463 (2008).
- S. M. Gartler, S. H. Waxman, E. Giblett, An XX/XY human hermaphrodite resulting from double fertilization. Proc Natl Acad Sci U S A 48, 332-335 (1962).
- J. P. Thiery, H. Acloque, R. Y. Huang, M. A. Nieto, Epithelial-mesenchymal transitions in development and disease. Cell 139, 871-890 (2009).
- L. Maya-Ramos, T. Mikawa, Programmed cell death along the midline axis patterns ipsilaterality in gastrulation. Science 367, 197-200 (2020).
- R. Hanayama et al., Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147-1150 (2004).
- S. Nagata, R. Hanayama, K. Kawane, Autoimmunity and the clearance of dead cells. Cell 140, 619-630 (2010).
- K. S. Ravichandran, Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med 207, 1807-1817 (2010).


 (1 votes)
(1 votes)