Replicating spinal cord development with microfluidics
Posted by cdemers, on 5 July 2016
Unraveling Development
Embryonic development is a complex and regulated spatiotemporal ensemble of signaling cues that control cell differentiation. Most of what we now know comes from experimenting directly on embryos. This provides biological realism, but involves a sea of uncontrolled/unobserved variables that sometimes obscures the basic underlying mechanisms. More recently, in vitro models, based on the differentiation of stem cells, have come into favor. These sacrifice some biological authenticity but have the distinct advantage of greater experimental ease and control over variables. Nevertheless, the culture dish or titer plate falls short of what could reasonably be considered an ideal imitation of an embryonic environment. As a result, in vitro models often give inconsistent or variable results.
Our goal has been to develop an experimental platform that captures some of the realism of in vivo models but with the ease and experimental control of in vitro methods. Our mantra is, “If you create the same physiochemical environment in vitro that occurs in vivo, cells will respond in the same way.” This not only includes the creation of molecular gradients and substrates, but the ability to alter these over time. To create this “ideal” in vitro environment, we’ve been using microfluidics.
A (very) brief history of microfluidics
Microfluidics is a growing field that deals with the fabrication of devices to control the flow and handling of small liquid volumes ranging from several hundred microliters to several picoliters. The actual physical size of any single microfluidic component can vary between several millimeters to several micrometers (about the size of a single cell) depending on the intended use, i.e. channels, valves, pumps, etc. .Examples of common microfluidic devices include: microbioreactors1, microsensors2, microanalysis3, lab-on-chip4, etc.
The field traces its origins to 1959 and the first demonstration of an integrated electronic circuit by Noyce and Kilby5. This discovery ushered in the microelectronics era and fueled the development of a plethora of high precision microfabrication technologies. Microfluidics “borrowed” these technologies to micromachine microstructures that comprise channels, valves and pumps. One of the first commercial applications of a microfluidic device was in 1979 when Hewlett-Packard invented the inkjet printer.
Because of its ancestral ties to microelectronics, most microfluidic systems were initially fabricated in silicon, but both technologies and materials have since expanded past their initial origins. More details of the history of silicon micromachining and microfabrication can be found here.
The role of microfluidics in developmental biology
Despite some setbacks, microfluidics are finally being applied to biology. Applications involving drug research and so-called “Organs-on-Chip” have enjoyed considerable attention. However, using devices to address fundamental biological questions has been slower to evolve. We find this rather surprising because developmental studies are particularly well suited to the strengths of microfluidics. Recent reports have revealed the incredible ability of embryonic stem cells to self-organize given appropriate instructive environment6-9. These are at present limited to bath applications of soluble factors. Microfluidic technologies can provide a much needed additional layer of control for more biologically relevant experiments.
This background was what prompted us to develop a microfluidic device that could mimic morphogen gradients. The device is comprised of a cell culture chamber flanked by supply channels reminiscent of how tissues are perfused by capillary beds. Soluble chemicals can freely diffuse into the cell chamber establishing long-term, stable gradients. Using this simple microfluidic device and only three morphogen gradients, Shh, BMP and RA, we were able to recapitulate much of the neural tube development including motor neuron spatial organization10. However, it is clear that not all neural types are represented in our little artificial spinal cord. Something is missing. Whether this is another morphogen or signal, electrical gradients or just wrong timings for the signals we’re using, the search should be extremely interesting. For example, the anteroposterior axis develops with its own morphogen gradients concomitantly with the dorsoventral axis. It’s likely that cells in the early spinal cord simultaneously integrate signals from both anteroposterior and dorsoventral axes.
We should be able to test this. The microfluidic device we have engineered is capable of creating almost any “designer” chemical landscape11 with any number of arbitrary morphogens. So there’s a whole set of experiments we are currently doing to explore some of these ideas. It’s also an example of how useful the technique could be to to developmental biology. All that is needed is an interesting question and a microfluidic design to test it. Although we haven’t yet faithfully replicated the physiochemical landscape in vitro, I think we’ve taken an important first step. I’m not sure where the journey will end but I’m hoping that we learn some interesting biology on the way. Fortunately, there is still plenty of research to be done.
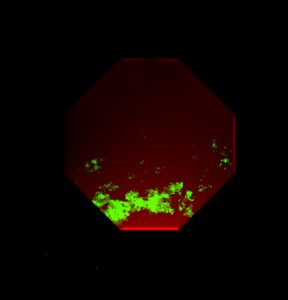
Figure 1: Mouse embryonic stem cells cultured in a four-port microfluidic device for 9 days. GFP expression indicates the presence of post-mitotic motor neurons, which clearly favor a defined spatial region as dictated by instructive cues supplied to the cells. Cells are superimposed with a red soluble fluorescent dye to illustrate the shape of the gradient formed in the microdevice. Overall chamber dimensions approximately 1.5mm at the widest point.
This work was performed at the University of Maine, MicroInstruments and Systems Laboratory (MISL), under a grant from the National Science Foundation, [IOS-1145949].
Our full paper can be viewed at here.
Press releases can be found from University of Maine and the Francis Crick Institute. The article was also briefly mentioned in Science.
1. Grünberger, A., Wiechert, W. & Kohlheyer, D. Single-cell microfluidics: opportunity for bioprocess development. Curr Opin Biotechnol 29, 15–23 (2014).
2. Erickson, D. & Li, D. Integrated microfluidic devices. Anal Chim Acta 507, 11–26 (2004).
3. Reyes, D. R., Iossifidis, D., Auroux, P.-A. & Manz, A. Micro Total Analysis Systems. 1. Introduction, Theory, and Technology. Anal Chem 74, 2623–2636 (2002).
4. Haeberle, S. & Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 7, 1094–1110 (2007).
5. Kilby, J. S. Miniaturized electronic circuits. (1964).
6. Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. & Brivanlou, A. H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Meth 11, 847–854 (2014).
7. van den Brink, S. C. et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242 (2014).
8. 3D Reconstitution of the Patterned Neural Tube from Embryonic Stem Cells. 1–34 (2014). doi:10.1016/j.stemcr.2014.09.020
9. Poh, Y.-C. et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun 5, 4000 (2014).
10. Demers, C. J. et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development 143, 1884–1892 (2016).
11. Smith, R. L., Demers, C. J. & Collins, S. D. Microfluidic device for the combinatorial application and maintenance of dynamically imposed diffusional gradients. Microfluidics and … 9, 613–622 (2010).


 (2 votes)
(2 votes)