Research Highlight: Decipher the Relationship between Cell Cycle Patterns and Fate Determination in Trunk Migratory Neural Crest Cells
Posted by Ruonan Zhao, on 29 June 2022
On June 8th 2022, a paper titled “Trunk Neural Crest Migratory Position and Asymmetric Division Predict Terminal Differentiation” was published on Frontiers in Cell and Developmental Biology. This is the second paper published by this research group on the topic of zebrafish trunk neural crest cell (NCC) migration in the past three months. Prior knowledge of their previous publication (Alhashem et al., 2022) is not required to understand or interpret this paper; however, I would like to prepare you with some general knowledge regarding the topic of neural crest migration/differentiation studied in this paper and mention a few key relevant findings from the previous paper.
NCC are an embryonic progenitor cell population that gives rise to numerous cell types and tissues in vertebrates. Pre-migratory NCC delaminate from the neural tube via epithelial to mesenchymal transition, following which NCC migrate throughout the embryo and undergo differentiation. NCC in the embryo trunk regions give rise to melanocytes, neurons and glia including Schwann cells and chromaffin cells. Migratory NCC streams consist of leader cells and follower cells. Leader cells initiate movement and remain at the front of the migratory NCC population to provide signals for the directionality of the population. Follower cells track leader cells’ movement and maintain cell-cell interactions necessary for migration.
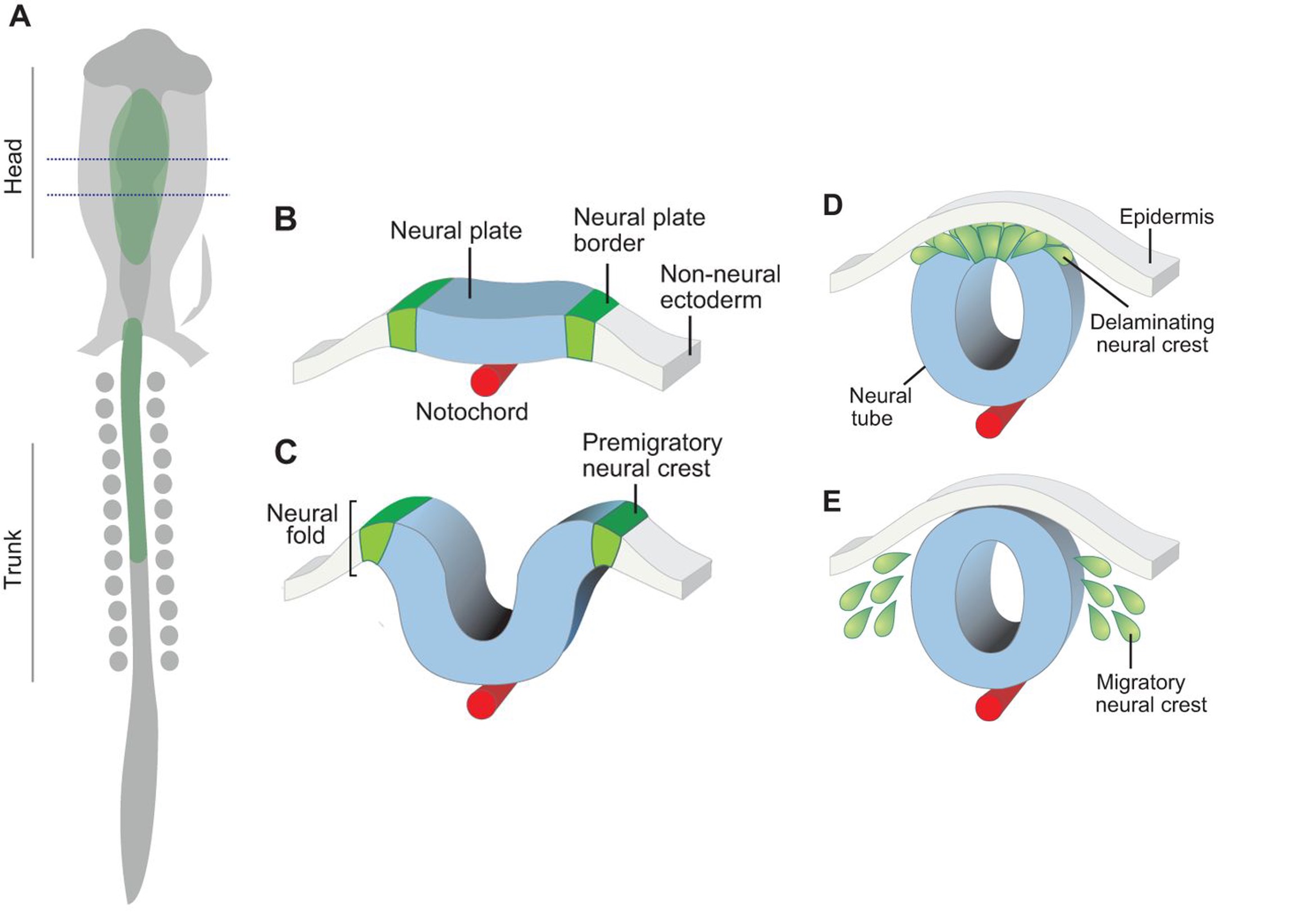
In Alhashem et al., 2022, researchers show that
- In each body segment, a single progenitor undergoes an asymmetric cell division to form a larger daughter cell, which later becomes a leader cell, and a smaller daughter cell, which migrates as a follower cell. The other progenitors undergo symmetric cell divisions to give rise to two follower cells.
- Leader and follower cells progress asynchronously through cell cycle during early migration: leader cells divide during migration at neural tube/notochord boundaries, but follower cells divide prior to migration initiation in the most dorsal region of somites.
- The total length of cell cycle is similar between leaders and followers during migration (no significant differences). However, leaders initiate migration during S phase, but followers initiate migration during G1 phase. Moreover, leader cells remain longer in S phase while followers spend longer periods of time in G1 phase in each cell cycle.
- Cell cycle progression is required for trunk NCC migration in zebrafish and is regulated by Notch signaling.
In the most recently published paper, researchers focus on later phases of trunk NCC migration when differentiation starts to occur. During migration, follower and leader cells maintain their relative positions within the migration chain. As a result, the position of migratory NCCs within a migratory chain correlates with their fates. When reaching the end of migration, leader cells divide perpendicular to the migration plane and give rise to a distal and proximal daughter cell. The distal cell remains at the most ventral position of the chain (lateral to the dorsal aorta) and differentiates to form the Sympathetic Chain Ganglia (SCG). The proximal cell stays dorsal to the SCG area and differentiates into Schwann cells. Interestingly, leader cell division is asymmetric so that the distal cell is bigger than the proximal cell sibling. On the other hand, followers divide parallel to the migration direction and their progenies populate the dorsal root ganglia (adjacent to the neural tube). In contrast to leaders, follower cell division is symmetrical generating two cells of similar sizes. Although it is beyond the scope of this study to address the mechanism regulating the pattern of cell division in leaders versus followers, it is discussed that an asymmetric distribution of Notch signaling in daughter cells after leader cell division could control identity allocation.
Another interesting observation demonstrated in the paper is that leader cells already express phox2bb, a noradrenergic marker, during migration (prior to reaching the destination for neuronal differentiation). This data suggests an early activation of transcriptional programs associated with lineage restriction in migratory NCC. How and when migratory NCCs become fate restricted has always been a major question in the field. The authors believe that this data supports the cyclical fate restriction model (Kelsh et al., 2021), which proposes that NCC can move between unstable sub-states biased towards specific differentiation outcomes. The transient differentiation biases can be determined by the expression of key fate determination receptors, the activity of the determination receptors, local environmental cues, and etc. Another recently published review paper also addresses and summarizes theories of NCC fate determination from existing single cell transcriptomics and multi-omics data (Erickson et al., 2022). It is worth a read if you would like to have a more up-to-date comprehensive view of this topic.
I personally enjoyed reading this paper because it demonstrates a strong link between two essential cellular activities: proliferation and differentiation. Cell division is so prominent during development that sometimes we don’t consider its possible interactions with other cellular processes. I also think most figures did a good job of illustrating the key points described in the texts. I especially like figure 4 and the 3D rendering that they did to show symmetric versus asymmetric division. It is a short and straightforward paper, but it leaves many intriguing topics to discuss and think about.


 (No Ratings Yet)
(No Ratings Yet)